Ion exchange is based on sorption, the transfer of solutes from fluid to solid particles. Undesired anions or cations from a liquid are exchanged for ions of the same charge within a resin.

Ion Exchange Theory
Ion exchange occurs on a resin. Resins are typically tiny particle-size solid beads. The picture on the left shows individual resin beads, and the picture on the right shows a variety of resins.


(Copyright Water Technologies Business Unit of Siemens Industry, Inc., Warrendale, PA)
The animation of ion exchange at the resin level shows the undesired ions from the liquid feed, in green, being exchanged for ions of the same charge, in blue, within the resin. The red co-ions are permanently bound in the resin.
Fixed Bed
Fixed bed ion exchangers are the most basic and most commonly used type of ion exchanger. They contain a packed bed of resin where the ion exchange takes place.

General Information
The ion exchange within a fixed bed ion exchanger occurs in a packed bed of resin, which is typically a solid, bead-like material. When the resin is exhausted, it is typically regenerated in a downflow manner.
Equipment Design
The resin beads are housed in vertical, cylindrical pressure vessels made of steel or rubber-lined stainless steel. Spargers at the top and bottom of the fixed bed ion exchanger distribute the liquid containing undesired ions (green) and support the resin. The resin contains desired ions (blue) of the same charge as the undesired ions, and permanently bound co-ions of the opposite charge (red). The undesired ions bind to the fresh resin, shown by the purple resin beads in the animation below, causing the desired ions to transfer to the liquid. Exhausted resin, shown by the green beads in the animation below, is depleted of desired ions and is regenerated by contact with a downflow of high concentration of the desired ions. If the column is operated in batch mode, it will run until equilibrium is reached.

New resin

Exhausted resin
Usage Examples
Fixed bed ion exchangers such as the ones to the left are used in waste treatment plants for chemical and water recovery. The ion exchanger on the right is used in an undergraduate teaching laboratory.
Advantages
- Requires less maintenance than other types of ion exchangers
Disadvantages
- Less effective ion exchange near-equilibrium
Mixed Bed
General Information/Equipment Design
Mixed bed ion exchangers have resins for both cation and anion exchange within the same column. The denser cationic and lighter anionic resins are mixed together by compressed air during loading, but separated during regeneration and backwashing. Regeneration is sequential; alkali solution is used for the anions, and acid is used for cations.
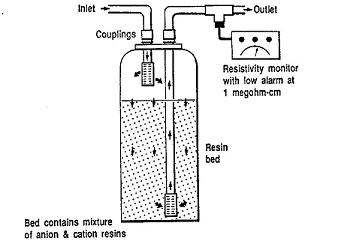
Usage Examples
Mixed bed ion exchangers are used to produce high purity water for various applications. For example, deionizers are used for boiler feedwater, process water, or rinse water applications; they use a cation resin that is a strong acid and an anion resin that is a strong base. The mixed bed ion exchanger shown below is used to remove residual ion traces in the feed stream from a demineralization process.

Advantages
- Produces water of a higher quality
Disadvantages
- High capital cost
- Complicated
Countercurrent
General Information/Equipment Design
Countercurrent ion exchangers are considered continuous process equipment, although the process is actually intermittent. The solution runs for a period of time, then stops to allow a slug of resin that is moved by the hydraulic impulse to flow countercurrent to the solution.
Usage Examples
Countercurrent ion exchangers are used in water treatment, distillation, and precipitation processes. Water is hardened by calcium and magnesium deposits. In water softening, Ca and Mg ions are replaced with other positive ions that do not contribute to hardness. The picture to the left shows demineralizes that produce high-purity water. The countercurrent ion exchanger shown on the right is used to extract gold and silver from leach solutions.
Countercurrent ion exchangers can also be used for nuclear applications. Extremely pure water is needed to cool nuclear reactors, to avoid corrosion and scaling. Since resins used for this purpose adsorb radioactive waste, they must be sealed and buried.
Advantages
- More effective than fixed beds for reactions close to equilibrium
Disadvantages
- No emergency standby for maintenance
- Costly
- Resin attrition, reducing efficiency
Continuous
General Information/Equipment Design
Continuous ion exchangers consist of two columns in parallel. Ion exchange takes place in one column, while the resin in the second column is being regenerated, resulting in continuous operation.
Usage Examples
Continuous ion exchangers are used when it is imperative that the product be continually generated. For example, metal contaminants are undesirable due to their color, taste, odor, or toxicity. In textile production, contaminating metals must be continuously removed for color uniformity and dyeing quality. Valuable metal recovery may also take place in ion exchangers: Metals are recovered from leach liquors that are derived from their ores. Ion Exchangers are also used to demineralize and decolor corn syrups.
Advantages
- No process interruption due to resin regeneration
- More effective than fixed beds for reactions close to equilibrium
Disadvantages
- More complex than fixed beds
Acknowledgements
- Aqua Technology, Pismo Beach, CA
- Eco-Tec Inc., Pickering, Ontario; now part of Koch Separations
- Eurodia Industrie SA, Rungis Cedex, France
- Fanta Equipment Company, Cleveland, OH
- Gekko Systems, Australia
- Remco Engineering, Ventura
- CA Water Technologies Business Unit of Siemens Industry, Inc., Warrendale, PA
References
- Bolto, B. A. and L. Pawlowski. Wastewater Treatment by Ion Exchange. New York: E. & F. N. Spon, 1987. Print.
- Dorfner, Konrad. Ion Exchangers: Properties and Applications. Ann Arbor, Michigan: Ann Arbor Science Publishers, 1972. Print.
- Nachod, Frederich C. Ion Exchange: Theory and Application. New York: Academic Press, 1949. Print.
- Perry, Robert H. and Don W. Green. Perry’s Chemical Engineers’ Handbook. 7th ed. New York: McGraw-Hill, 1997: 16-56 – 16-66. Print.
Developers
- Sujata Naik
- Alex Wozniak
- Kelsey Kaplan
- Steve Cotton


