Pharmaceutical processes start with the production of the Active Pharmaceutical Ingredient (API). This consists of feed handling, reactions, recovery, and drying. After these steps, a pure API is ready to be used in pharmaceutical products to deliver medicine to the body. The following sections describe these steps and equipment relevant to API production.
Pharmaceuticals Process Map
Feed Handling
The first step of API production is feed handling, which is the preparation and transport of raw materials used for subsequent reactions. Throughout the pharmaceutical manufacturing process, materials are stored in intermediate bulk containers (IBC), which are normally drums. Drums are used to transport and store the bulk feed and to store liquids that are used as reactants, solvents, and catalysts. Solids that are used as reactants can be temporarily stored in plastic drums or sacks. To begin the process, materials are often transferred to a mass flow hopper so that the feed can be accurately measured. When liquid materials are transferred, pipes are used to prevent contamination. Contamination is also prevented by using conveyors to transport solids between steps.
Reactions
After the feed materials are prepared, chemical reactions convert the feed into the API. The production of API can occur by chemical synthesis or by using biotechnology, depending on the API chemistry.
Chemical Synthesis
In industry, typical reactors used to achieve a chemical synthesis of API include batch reactors, loop reactors, and specialized batch autoclaves.
Batch Reactors
Batch reactors, like the one shown below, can range in size from 500 liters to 16 cubic meters depending on the scale of production for the API. Since contamination is a major concern in the pharmaceutical industry, these reactors are normally made from stainless steel or glass-lined mild steel and are fitted with a manway for easy cleaning. Reactions can be carried out at temperatures ranging from -25°C to 160°C and the pressure can vary from a full vacuum to 6 atm. These reactions are very sensitive to the environment, especially temperature changes, which can cause the product to be contaminated. To prevent contamination external jackets or pipe coils are fitted on the reactor to control the temperature. The coil or jacket usually contains a fluid that transfers the heat to the reactor. Agitation is also used in the reactor to provide mixing and heat transfer.
Loop Reactors
A loop reactor is a variation of a continuously stirred tank reactor, which is used for gas-liquid reactions at high pressures. Of the three types of loop reactors, jet, gas lift, and propeller, the jet loop reactor is the most common in chemical synthesis. This is because this type of loop reactor has higher heat and mass transfer rates and is easier to scale up. The jet loop reactor operates by injecting a heated, pressurized, liquid with a catalyst through the top or bottom of the reactor. The liquid is then fed through a loop inside the reactor where it interacts with the reactants to begin the reaction. The products that form gather at the bottom of the loop, where they are released from the system. As the liquid evaporates, the catalyst is recycled to a small tank where it is diluted and fed back into the reactor.
Batch Autoclave
A batch autoclave is a special type of batch reactor that is used for high-pressure reactions or for feeds that are solids or a slurry. These reactors can operate above 5,000 psi and 500°C and are normally equipped with a magnetically driven stirring system to ensure even heat exchange. They also have computer-operated sensors and valves so that the whole system can be monitored from behind a blast wall, given the extremely high pressures. When heating is needed they can be equipped with a heating element.
Biotechnology
Bioreactions mimic reactions that occur in living organisms. An example of biotechnology used in API production is fermentation, in which a fermenter is used as the reaction vessel.
Fermenters
Fermenters can be used in API manufacturing to produce antibiotics through microbial fermentation. The process is characterized by fast growth rates, which can lead to mass and heat transfer issues. To account for this, often a stirred tank or sparged tank fermenter is used. A stirred tank uses a mechanical agitator to mix the components of the reaction to aid the mass and heat process. A sparged tank does not use a mechanical agitator but uses a gas stream to circulate the reactants in the fermenter. Both of these reactors undergo aerobic fermentation and therefore can only be used in aerobic API processes. For anaerobic processes, an open tank design is used since the microorganisms produce a foam covering of carbon dioxide which blocks the oxygen from the reactants. An example of a fermentation system is shown below.
Recovery
After pharmaceutical reactions occur, the exit stream can contain API, unreacted feed materials, solvents, and any additional reaction additives. Typical recovery methods to separate API from reaction byproducts are described below.
Distillation
Distillation is a thermal process used to separate liquid species by evaporation. Batch, flash, and fractional distillation columns are used in API processing. In batch distillation, the composition of the material inside the column varies as the process continues and the volume drops. As a result of the volume drop, there is a loss of heat transfer surface, which causes the efficiency of the equipment to decrease. To counter this drop in heat transfer surface, a heat exchanger is often used to provide an external surface as the product is pumped through it. Flash distillation is a process that uses a single-stage column with one material while fractional distillation uses multiple materials. Often vacuum pumps are attached to the distillation columns so that evaporation can occur at lower temperatures since often materials in API processing can be heat sensitive.
Membranes
In the pharmaceutical industry, pressure-driven processes often employ membranes. One of the ways membranes are used in this industry is by direct flow filtration. This involves passing the feed solution through a membrane, where particles are retained by physical capture or absorption. These membranes are commonly available in cartridges that contain the membrane or can be found as disposable capsules. The main problem with direct flow membranes is that over time they get built up with particles that reduce the effective membrane area and can cause contamination of the drug if not addressed. One way to overcome these factors is by using tangential flow filtration. In this process, the feed stream runs parallel over the membrane to separate the particles. These membranes can be cleaned more easily and have longer lifetimes than membranes used in direct flow filtration.
Crystallization
The final API is often in the solid stage in most synthetic processes. Crystallization is a process that includes a variety of steps for solid isolation, such as cooling, evaporation, concentration, reaction precipitation, pH change precipitation, and solvent change precipitation. These actions are performed using a batch crystallizer, which is a modified batch reactor. This crystallizer allows agitation and heat transfer control to avoid damage to the final solid product. Often materials of the desired crystal type are added at the appropriate time to initiate the processes in the direction of the desired final product. This process can also be carried out with a continuous crystallizer, but this is less common in API manufacturing.
Filtration
After a solid is produced it can be separated from the liquid by filtration. A calmic or bag filter can be used to remove small quantities of solid but for larger quantities, a nutsche filter is used. The original single sheet nutsche filter was simply a box that had a vacuum attached at the bottom to draw the filtrate through the filter medium. The problem with this design is that there is nothing to protect the operator from the contents inside the box as well as protecting the contents from contamination. For this reason, an agitated pressure nutsche filter is more common in API manufacturing. This design uses a pressurized chamber above the filter medium where the filter cake is smoothed using an agitation arm. A vacuum is used to pull the filtrate out of the cake or pressure can be applied to the top to create a pressure difference. After the filtrate is removed the cake is washed inside the filter, so that the vacuum can pull it out of the cake, to remove soluble impurities. From this step, the solid cake can be removed from the filter for drying. Sometimes the nutsche pressure filter can be used to dry the cake as well by passing hot water through coils along the body of the filter. This way the material does not have to risk contamination being transported to a separate dryer, but this process can be a time-limiting factor for the plants.
Centrifugation
The principle of a centrifuge is to create enough centrifugal force to push the liquid through a filter medium that separates it from the solid. Four types of centrifuges are used in API manufacturing. The vertical axis with a top discharge centrifuge is mainly used for small quantities because the operator can get exposed to the material when discharging it by removing a basket. The other vertical axis centrifuge uses a plough to push the material out of the basket and into a chute at the bottom. The horizontal axis centrifuges can spin at higher speeds than the vertical axis centrifuges and are often preferred since they provide a higher centrifugal force to drive the liquid out. Ones that use a peeler discharge the solid by using a peeler blade that pushes the solid into a chute. The inverting bag discharge removes the solid by simply inverting the filter medium to create a bag.
Drying
After recovery of the API from reaction byproducts, the API may still be wet with reaction solvent. Dryers are used to vaporize the solvent and produce a dry powder of API. Drying the API into dry powder form can be vital to accurately dose some pharmaceutical products, such as tablets or capsules. The type of dryer used depends on the pharmaceutical being produced and can include fluid bed dryers, tray dryers, rotary dryers, and filter dryers.
Fluid Bed Dryers
In a fluid bed dryer, heated gas is blown through a filter cake, evaporating the solvent. This gas stream has to be passed through a filter to prevent contamination of the solid. The dryer uses a perforated basket to hold the filter cake and to allow gas to pass through. While the basket can be filled by hand, gravity-fed processes are preferred to reduce the chance of product contamination.
Tray Dryers
Thin layers of filter cake are placed atop trays inside a tray dryer to evaporate the solvent. Heat is provided by a single fluid system inside of a jacket that operates at temperatures of 40°C – 100°C. Liquid ring pumps or dry vacuum pumps generate a vacuum inside of the tray dryer. In most pharmaceutical plants a dust filter is fitted on the dryer to prevent contamination from the outside. The problem with this type of dryer for many active pharmaceutical ingredients is low efficiency, messy loading, and that hard lumps of the API can form on the trays.
Rotary Dryers
Rotary dryers are the most widely used in manufacturing the API because of their high efficiency, resulting in low drying times. These dryers consist of a horizontal cylindrical chamber and are often fitted with a heating and cooling jacket. They have a slow paddle that rotates the solid continuously to enhance heat exchange. The heating jacket also has to have a high surface area to volume ratio for the most efficient heat exchange.
Spray Dryers
All spray dryers used in pharmaceutical processes pass the gas through a filter to ensure that it is clean when it comes in contact with the drug. The outlet gas is also passed through a filter so that the rest of the plant is not contaminated. Nitrogen or air is used as the drying gas when the drug is recovered using inorganic solvents. Nitrogen is used to dry organic solvents in a closed configuration. The temperature, pressure, and other operational parameters depend on the solvent being dried.
Filter Dryers
A filter dryer combines a nutsche filter with a tray dryer to combine the filtering and drying steps. The nutsche filter operates as usual but is fitted with a heating jacket and vacuum pump that dry the material, leaving a dry powder of the API.
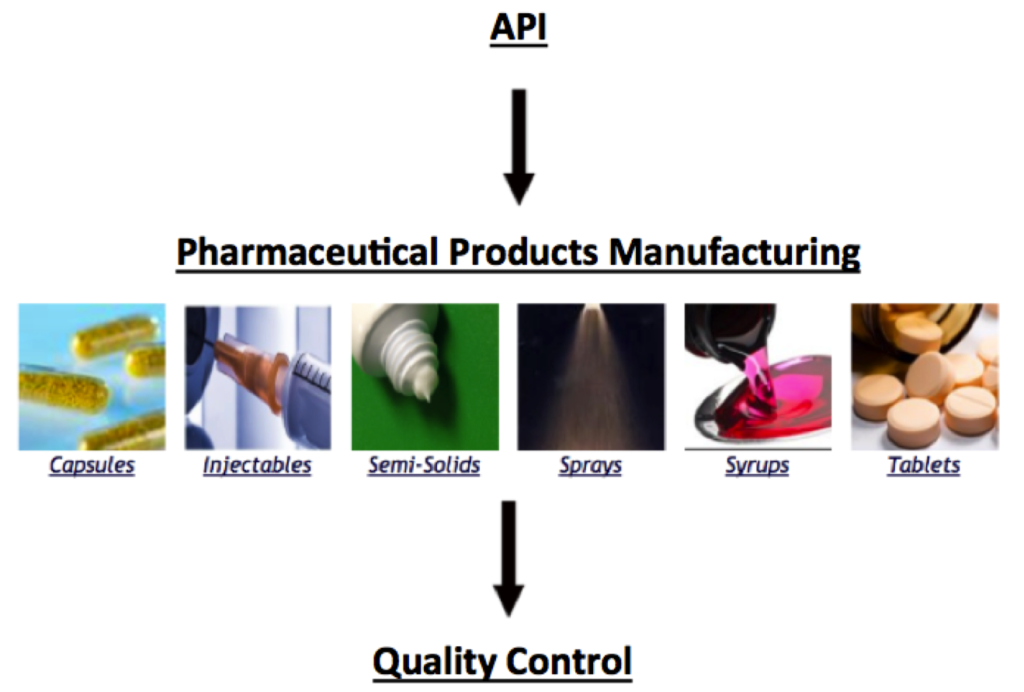
After the production of pure API, the next step of the pharmaceutical production process is to insert the API into a pharmaceutical product specific to the desired delivery method. Pharmaceutical products include tablets, capsules, semi-solids, syrups, injectables, and sprays. The linked modules describe the respective processes and equipment used to incorporate API into pharmaceutical products that are safe for human consumption.
Acknowledgements
- Alfa Laval, Richmond, VA
- Central Fabricators, Cincinnati, OH
- GEA Process Engineering Inc., Columbia, MD
- Pfaudler, Inc. , Rochester, NY
- Powder Systems Limited. , England no. 2233044
- PRAB, Kalamazoo, MI
- R. Simon (Dryers) Ltd., Nottingham, UK
- Sulzer Chemtech Ltd. , Switzerland
References
- Bennett, Bill, and Cole, Graham. Pharmaceutical Production: An Engineering Guide. United Kingdom: The Institution of Chemical Engineers, 2002. Print.
- “Batch Autoclave Reactor Systems.” Energy and Environment Research Center. University of North Dakota, 1992. Web. http://www.undeerc.org/.
- “Chemical Reactors.” CIEC Promoting Science at the University of York, York, UK. University of York, 18 Mar. 2013. Web. http://www.essentialchemicalindustry.org
- Doraiswamy, L. K., and Deniz Uner. Chemical Reaction Engineering: Beyond the Fundamentals. United States: CRC Press Inc, 2012. Print.
- Ende, David J. Chemical Engineering In the Pharmaceutical Industry: R&D to Manufacturing. Hoboken: Wiley, 2010.
- Pabby, Anil Kumar. Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, And Biotechnological Applications. Boca Raton: CRC Press, 2009.
- “Pharmaceutical Spray Dryers – PHARMASD™.” GEA Pharma Systems. GEA Process Engineering, Web. https://www.gea.com/en/index.jsp.
- Wang, H.Y, University of Michigan, personal communications, 2015-2017.
Developers
- Thomas Plegue
- Howard Hsu
- Nathan Hoffman
- James Rivard