This section discusses the basic science of polymers and polymerization reactions as well as how polymers are produced in an industrial setting. There are a variety of ways to form polymers, but the equipment used mainly depends on the properties of the polymer.

Polymer Manufacturing Steps
Making the polymer is the first step in the manufacture of polymer products:
Polymerization Reactions
Polymerization reactions involve three basic steps: initiation, propagation, and termination. An initiation reaction must occur to functionalize a monomer unit before the reaction can begin. Once a monomer has been functionalized, another monomer unit can bind to it, and the functionalized carbon center propagates to the new monomer unit. In this fashion, monomer units continue to attach to the polymer chain until termination occurs. There are many ways to stop a polymerization reaction, the most applicable being for two propagating chains to collide and bind together. Alternatively, a functional center on a monomer could transfer its free electron to a new monomer unit to end the chain or bind to a non-polymerizing contaminant.
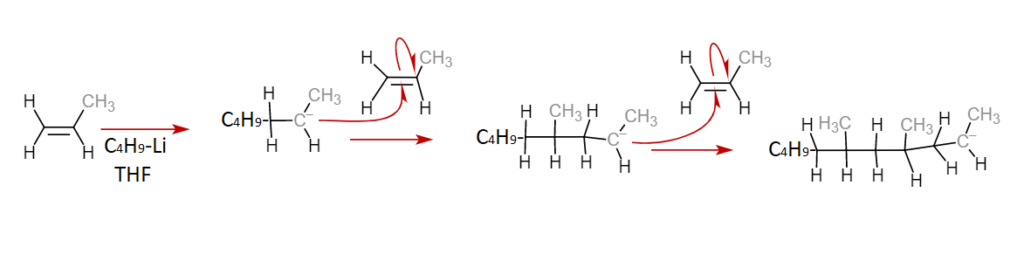
Anionic Polymerization
This form of addition polymerization involves the opening of a double bond by a negatively charged ion. A new monomer binds to this site, shifting its own electrons and opening another negatively charged site. The polymerization terminates when the propagating end binds to a new species that is unable to propagate further. Anionic polymerization is characterized by its low polymerization temperatures and its high polymerization rates. Synthetic polydiene rubbers, used in tires, can be produced in this manner. Below is an example of anionic polymerization, note the presence of the negatively charged carbon anion in the propagation phase.
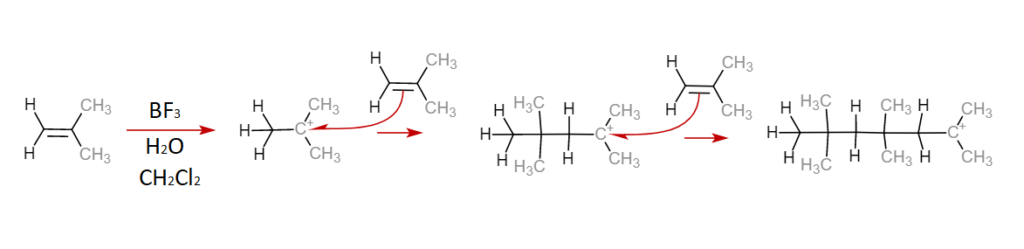
Cationic Polymerization
This form of addition polymerization is very similar to anionic polymerization but involves positively charged binding sites as opposed to negatively charged ones. A double bond on a monomer is opened by a carbonium ion, a positively charged carbon atom, and the binding site propagates down the chain as additional monomer units bind to the chain. Typically, as temperatures decrease, the rate of polymerization and the degree to which a chain has polymerized increase. Cationic polymerization is used to form polyisobutylene, a key component of inner tubes. Below is an example of cationic polymerization, note the presence of the positively charged carbon cation in the propagation phase.
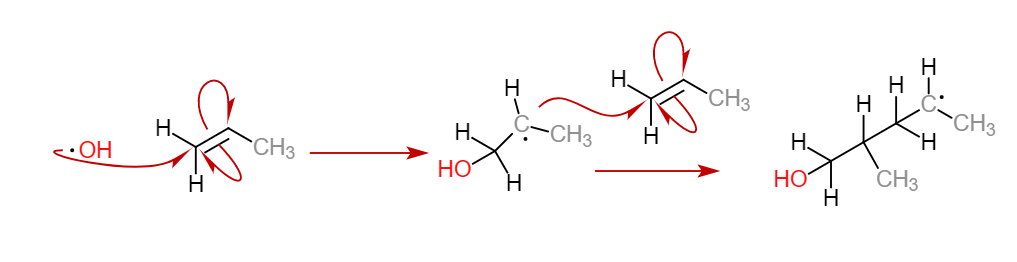
Free Radical Polymerization
This is the predominant polymerization reaction. When a free radical is generated as a byproduct of a reaction, it can interact with a monomer and open a double bond on it, allowing a new unit to bind. The binding displaces a new radical at the end of the new monomer and thus the polymerization can continue. Researchers have used this polymerization method to adhere polymers to the walls of carbon nanotubes to alter the physical properties of both components. Below is an example of a free radical polymerization, note the presence of the free electron radical in the propagation phase.
Step-Growth Polymerization
This form of polymerization is also referred to as condensation polymerization, due to the generation of water as a byproduct. In this reaction, functional groups on two monomer units react and bond, producing a water molecule in the process. It is referred to as step-growth because each reaction between functional groups on two monomer units is independent of another reaction along the chain, unlike the three previous polymerizations, which depended on the propagation of free radicals or charged centers. Nylon is produced using a step-growth polymerization reaction.
Types of Polymers
There are two basic forms of polymer, thermosets, and thermoplastics, which are distinguishable by their physical properties. Thermoset polymers have undergone cross-linking and cannot change shape under heat, while thermoplastics have tangled, branched chains that, when heated, can reshape the polymer as a whole.
Thermosets
Thermoset polymers undergo cross-linking, or a chain extension polymerization reaction wherein multiple chains can form bonds with one another, causing the final, cured polymer to be very rigid and unmalleable. Therefore, the polymer will not flow when heated. Before curing, thermosets are typically viscous, liquid resins. As such, a thermoset compound must be cured in a mold to attain the desired shape and characteristics, since it cannot be reshaped. In some cases, some thermoplastic chains can be cross-linked together to form a thermoset.
Thermoplastics
Thermoplastics differ from thermoset compounds in that thermosets rely on cross-linked bonds between chains whereas thermoplastics are bound by intermolecular forces and tangling between branched chains. The character of a thermoplastic polymer is determined by 3 factors: molecular weight distribution, molecular orientation, and degree of crystallinity. Unbranched and linear thermoplastics form dense crystals and tend to be very brittle, whereas highly branched polymers don’t often form crystal structures and are thus much rubberier. The molecular weight distribution within the thermoplastic determines its liquid-phase viscosity. When heat is applied to thermoplastics, the forces holding the branches in place become weakened, and the polymer can be deformed and reshaped.

Feed Handling
The first step in the production of a polymer product is to prepare and transport the feed to the reactor. Many bulk solid reactants can be purchased in bag form and are later stored in intermediate bulk containers (IBCs) within a production facility. Liquids are typically held in drums until use. To create a dry mix of reactants, all powders and solids are added together in an IBC in the correct stoichiometric quantities and tumbled together before being emptied into a mass hopper that feeds the reactor. Liquid mixtures must undergo mechanical mixing to adequately dissolve any solid reactants. Liquids are typically fed and conveyed via piping to reduce the risk of contamination. In the case of batch reactions, reactants are typically added manually to a stirred tank reactor where they are mixed prior to the reaction.

Polymerization Reactors
After the feed is mixed, it is fed into a polymerization reactor where the polymer-forming reaction occurs. Polymerization reactors can be operated in both continuous and batch modes. Continuous polymerization reactors have not been applied to a wide variety of processes, but those polymers that are produced continuously make up a majority of total industry output. Batch reactors are typically used for all other consumer orders, as they allow for special orders and polymer modification.
Continuous Polymer Production
Continuous polymerization reactions typically occur in single or multi-tubular reactors with plug flow conditions or a stirred tank reactor to achieve perfect mixing and heat distribution. To learn more about the general applications of plug flow reactors and continuous stirred tank reactors, see the PFR page and the CSTR page.
Continuous Stirred Tank Reactor
These polymerization reactors consist of a tank with an inlet for the monomer mixture stream and an outlet for the polymer stream. An impeller driven by a motor typically mounted in the head plate stirs the material in the reactor. When designing a continuous stirred tank reactor, perfect mixing is typically assumed, but this is not always the case in polymerization systems due to the low diffusivity of polymer particles and increasing viscosity of the mixture, leading to increasingly poor overall mixing. Thus, it is normal to see an overall polymer conversion in a continuously stirred tank reactor of about 85%. Because of this, it may be necessary to separate unreacted components or feed the product stream into a post-treatment tubular reactor to finish the reaction.
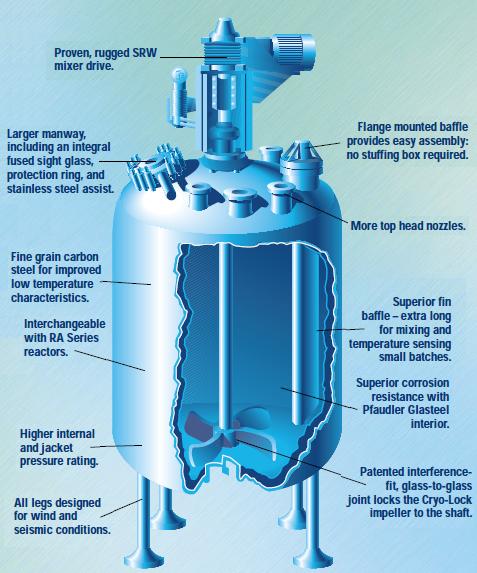
Tubular Plug Flow Reactor
These are perhaps the simplest form of polymerization reactors. The monomer reactant mixture is forced through a pipe where the reaction occurs along the pipe length, and as polymers tend to be very viscous, the system can be assumed to be in the laminar regime. Ideally, monomers in a theoretical slice of the stream travel at the same velocity in plug flow and interact with each other to react and form the polymer, however, this can be somewhat inaccurate, depending on how the mixture behaves near the pipe wall. As previously stated, as the polymerization reaction occurs it generates a good deal of heat which must be removed by a cooling mechanism, typically a cooling jacket or water stream.
Issues can arise as the diameter of the pipe is changed. It is possible that the transfer of heat from the center to the edge of the pipe, where the energy is removed, might not occur fast enough, leading to the possibility of thermal runaway. Furthermore, the diffusion of monomer particles along the plug can also be a limiting factor due to viscous resistance from the forming polymer. As a way to mitigate these issues while still increasing the flow rate, it’s possible to instead use multiple smaller-diameter pipes as a multi-tubular reactor.

Batch Polymer Production
To learn more about batch reactors, visit the batch page. In batch production, all the polymer reactants are added through ports in the top of the kettle and the mixture is continuously stirred. Typical polymer batch reactors are made of stainless steel or glass, with capacities ranging from 5 to 30,000 gallons. Batch production can sometimes be difficult due to heat transfer limitations of the polymerization. It may be necessary to use a cooling jacket to effectively displace heat away from the kettle and prevent thermal runaway due to an exotherm, where the polymerization rate is at a maximum. Batch-to-batch variability can also be problematic. Reactions creating cross-linking compounds are best performed using batch reactors since viscosity increases over the course of the reaction and the polymer might otherwise get stuck in a tubular reactor.
Separations
The polymerization reaction is often followed by a separation step to remove unreacted monomer units, solvents, contaminant particles, and byproducts. This stage is often the most economically substantial.
The separation method used depends primarily on the differences in physical properties between two components. If solid particles are present, sometimes it is possible to wash the polymer with an aqueous or sometimes non-aqueous solution. Alternatively, if the polymer is viscous enough, filtration can be used to remove large solid particles. In the case of entrained liquids within the polymer system, a coalescing filter can be used. The polymer flows through the filter and the small pockets of liquid coalesce on the fibers of the filter. See the diagram of the coalescing filter below.

In some cases, it is possible to precipitate the polymer out of a solution, though this can prove problematic. The polymer must be dilute enough in solution so that it precipitates into small particle-size clumps, which can be easily dried to remove the solvent. If the polymer concentration in a system is too high, the coagulated particles can be much larger and the solvent can become trapped within a particle.
Wiped-film devolatilizers and flash devolatilization can be used to remove volatile compounds from a system. The principle relies on the diffusion of the volatiles out of the polymer and into the air driven by a concentration gradient between the two mediums. However, due to the viscous nature of polymers, the diffusion-limited mechanism can often be time-intensive. It is possible to reduce the time requirements of the process by increasing the interface area between the polymer and the medium into which the volatile diffuses. A flash devolatilizer increases the surface area of the polymer by drastically reducing the pressure in a system, causing volatile component bubbles to form within the polymer. A wiped-film devolatilizer wipes the polymer along a large surface area drum to increase the area for diffusion of the volatile out of the polymer.

Acknowledgements
- Lyle Industries, Inc., Beaverton, MI: part of BMG solutions
- PRAB, Kalamazoo, MI
- Robert Hesketh, Rowan University, Glassboro, NJ
- Alfa Laval, Richmond, VA
- Perma Pure LLC, Lakewood, NJ
- Pfaudler Inc. , Rochester, NY
- Milacron, Batavia, OH
References
- Nauman, E.B. McGreavy, C. Schork, F.J. Polymer Reactor Engineering. 1st Edition. New York, NY. Springer Science and Business Media, 1994, Print.
- Griskey, Richard G. Polymer Process Engineering. 1st Edition. New York, NY. Springer Science and Business Media, 1995, Print.
- Schork, F.J. Deshpande, P.B. Leffew, K.W. Control of Polymerization Reactors. 1st Edition. New York, NY. Marcel Dekker, Inc. 1993, Print.
Developers
- Joel Holland