Pharmaceutical capsules are an alternative to tablets when compressed solids cannot be formed. Capsules use a gelatin barrier to deliver the active pharmaceutical ingredient (API) and additional inactive ingredients to the body. The barrier is used to control the release time and can mask an unpleasant taste of the pharmaceutical formulation. There are two types of pharmaceutical capsules, hard-shelled and soft-shelled. Hard-shelled capsules, shown below, are typically used to deliver medicinal powders or granules to the body, whereas soft-shelled capsules are mostly used to deliver liquid formulations.
This module describes the production equipment used in both manufacturing processes. The Quality Control module describes additional equipment used to monitor quality parameters during these processes.
Pharmaceuticals Process Map
Hard-Shelled Capsule Manufacturing
Hard-shelled capsules are typically used to deliver solid powder or granules of medicine to the body. However, some hard-shelled capsule products do contain liquid formulations.
The manufacturing of hard-shelled capsules varies for each pharmaceutical product in the order and need for certain process steps. However, these processing steps are standard for most products: mixing of API and inactive ingredients, processing into a solid formulation, capsule filling, and capsule finishing. The following sections describe these processing steps and related equipment.
Mixing
Mixing is used in the manufacturing of hard-shelled pharmaceutical capsules to combine the API and other inactive ingredients into the formulation to be delivered to the body. Examples of common mixers found in capsule manufacturing processes include tumblers and ribbon mixers.
Tumbling mixers, or tumblers, are the most commonly used mixer for hard-shelled capsule processes. They are used to gently mix solid API and other ingredients before capsule filling. Tumblers are widely used because their batch operation allows for close quality control and their gentle mixing prevents damage to fragile powders or granules, such as the API after solids processing.
In hard-shelled capsule manufacturing, tumblers are typically found in twin-shell, cubic, or cylindrical shapes. The visual below shows a cylindrically shaped tumbling mixer in action.
Ribbon mixers are a widely used convective mixer for powders, such as powdered API and ingredients found in hard-shelled capsules. They are advantageous in that ribbon mixer impellers can scrape the outside wall of the mixer to prevent accumulation and promote mixing, as shown below. These mixers can be adapted for both continuous and batch processes, where a tumbler is limited to batch processing.
Solids Processing
In almost all hard-shelled capsule manufacturing processes, the API and other inactive ingredients are combined into a solid formulation to fill a capsule. It is common that the solid ingredients need to be processed before and/or after mixing. The types of solids processing that may occur include granulation, sieving, milling, and drying. These processes are vital in some processes to obtaining the correct dosage. The following processes and common equipment are described below.
Granulation is the process of producing granules, small compact particles of a substance. In many hard-shelled capsule formulations, granules of the API, or mixtures of ingredients are produced. This is done to provide uniform particle size and is vital in some processes in obtaining the correct dosage before filling capsules. A common type of granulator used in hard-shelled capsule manufacturing is a mixer granulator, shown below.
Sieving is used in hard-shelled capsule manufacturing to separate powders or granules of pharmaceutical ingredients by particle size. Common sieving methods include using a sieve stack, shown below, which contains openings of a particular size. The powder or granules are fed to the sieve stack and are shaken until particles pass through the sieves that have large enough openings. This separates particles into ranges of particle sizes for which the weight of the material can be accurately calculated. In many hard-shelled capsule processes, this is vital before mixing the ingredients or capsule filling to accurately dose each capsule.
Milling is used in hard-shelled capsule manufacturing to crush solid granules into powder. This may be required before mixing to crush API granules to incorporate better mixing with other powder ingredients. It may also be used if granules of ingredients are mixed first to crush the mixture particles. Milling provides uniform particle size, which is essential for proper dosage. A cone mill is commonly used in pharmaceutical processes, shown below.
In some hard-shelled capsule manufacturing processes, liquid can be introduced to the process at the mixing or solids processing steps. As a result, drying is required to remove excess moisture. This is important in obtaining the correct dosage for capsule filling as an unknown amount of moisture can drastically change the concentration of other ingredients. Common types of dryers in pharmaceutical processes include rotary dryers, fluidized bed dryers, spray dryers, conveyor dryers, and thin-film dryers.
Capsule Filling
After mixing and potentially other solids processing steps, the capsule is then filled with the pharmaceutical formulation. Hard-shelled capsules enter the process in two parts, called the cap and body. See the image below for a visual of a hard-shelled capsule in two parts.
During capsule filling, empty capsules and the solid formulation are simultaneously fed to a capsule filler, shown below, through different transfer lines. Inside the equipment, the body is filled with the formulation at the correct dosage. Next, the cap is applied to the top of the body, completing the capsule filling process. Capsule fillers are highly specialized equipment and vary in design and level of automation for each manufacturer. However, they follow the same basic process described above.
Capsule Finishing
After capsule filling, the hard-shelled capsules will be finished by polishing and dedusting the capsules by cloth dusting and brushing to remove any residual powder or granules on the outside surface. This is done to prevent any unintended exposure of the pharmaceutical to the processing operators or consumers.
At this point, the capsules are ready for testing against product specifications. The Quality Control module describes equipment relevant to this testing process.
Soft-Shelled Capsule Manufacturing
Soft-shelled capsules, or soft gels, are typically used to house liquid, non-aqueous API, and other ingredients. The capsules contain gelatin as well as glycerol to maintain flexibility.
The process steps to manufacturing soft-shelled capsule pharmaceuticals include mixing and encapsulation. The following sections describe these steps and the common equipment used for the respective steps.
Mixing
For soft-shell capsules, the API is typically a liquid. Additional inactive ingredients may be necessary for the final pharmaceutical formulation. Several types of mixers may be used to combine the liquid API with the inactive ingredients, depending on the inactive ingredients and process conditions.
Encapsulation
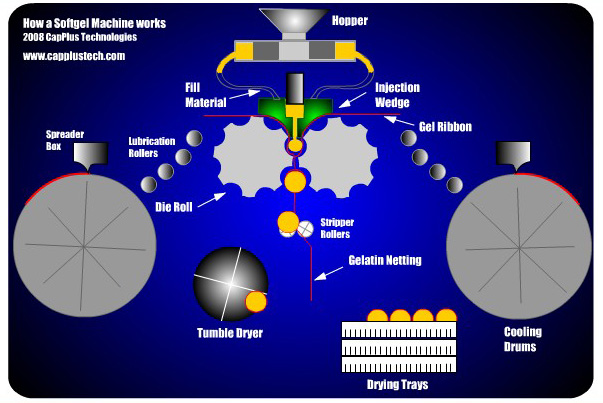
Encapsulation, the process by which the pharmaceutical formulation is added to the soft-shelled capsule, is accomplished using a rotary die that creates and fills the capsule in one step. In this process, two ribbons of the capsule material, gelatin, and glycerol, flow together and enter the rotary die on either side. At the same time, the pharmaceutical formulation is injected into the die between the two ribbons to fill the capsule. The capsule takes the shape of the rotary die, typically spherical or elliptical. After injection, the gelatin and glycerol ribbons are sealed by pressure and heat to complete the capsule. The capsule is then cut from the ribbons and is collected below the die. An image depicting the rotary die encapsulation process is shown below.
At this point, the capsules are ready for testing against product specifications. The Quality Control module describes equipment relevant to this testing process.
Acknowledgements
- GEA Process Engineering Inc., Columbia, MD
- Glen Mills, Inc., Clifton, NJ
- H.C. Davis Sons Manufacturing Co., Inc., Bonner Springs, KS
- Mendel Company, East Hanover, NJ
- Nicomac Inc., Norwood, NJ
- W.S. Tyler, Mentor, OH
- CapPlus Technologies, Phoenix, AZ
References
- Bennett, Bill, and Cole, Graham. Pharmaceutical Production: An Engineering Guide. United Kingdom: The Institution of Chemical Engineers, 2002. Print.
- Cole, Graham C. “The Design and Operation of a Facility for Filling Hard Shell Gelatin Capsules.” Capsugel Library, 1999. Web.
- Keenan, Thomas R. “Gelatin.” Kirk-Othmer Encyclopedia of Chemical Technology. Wiley, 2003. Print.
- Parm, Kinam. “Solid Dosage Forms: Capsules.” Web.
- “Pharmaceuticals.” Kirk-Othmer Encyclopedia of Chemical Technology. Wiley, 2007. Print.
- Niazi, Sarfaraz K. Handbook of Pharmaceutical Formulations. Vol. 2. CRC LLC, 2004. Print.
- Trottier, Remi. “Size Measurement of Particles.” Kirk-Othmer Encyclopedia of Chemical Technology. Wiley, 2007. Print.
Developers
- Nathan Hoffman