Stripping columns are used to remove unwanted materials from process streams. The air stripping column shown below is used to remove ammonia from water and wastewater feedstreams. This section describes various types of air stripping columns as well as steam strippers.

Farmingdale, NY)
Air Stripping Columns
Air stripping columns are most widely used for the removal of volatile organic compounds (VOCs) from water. By forcing air through contaminated water, the VOCs are evaporated from the water and the air passing through the column absorbs the contaminated vapor. The air can then either be removed or pumped into the atmosphere if the concentration of VOCs in vapor form is low enough. The most common types of air strippers are packed-column air strippers and sieve-tray air strippers, though diffused- aeration tanks can often act as an effective means of air stripping as well.
The design of air stripping columns is governed by differences in volatility between the volatile organic compounds (VOCs) in the contaminated water. The more volatile components transfer from the water to the air stream. The air exits the top of the column and contains the most volatile components, while the least volatile components exit with the water out the bottom of the column.


(Copyright Monroe Environmental
Corporation, Monroe, MI)
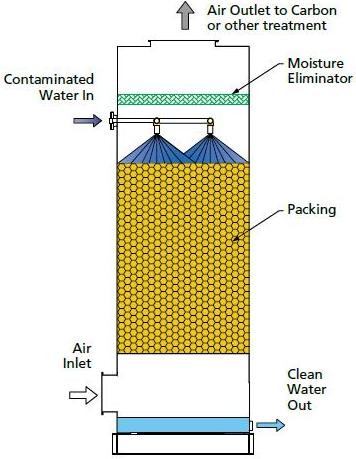
Packed-Column Air Strippers
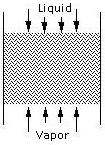
In these strippers, water enters from the top of the column and is misted over the porous packing material while air is blown from the bottom of the column. As the water and air meet, most of the VOCs are transferred to the vapor phase and removed from the top, while the treated water exits through the bottom of the column.
Equipment Design
After the contaminated water enters the column, it flows down the column and through the packing countercurrent to the air stream. The packing serves to increase the amount of contact between the two streams and enhances the stripping as a result. After leaving the column, the air is either distributed into the atmosphere or is collected for purification.
Packing types include dumped packing and stacked packing. The packing channels the liquid flowing from the top to the bottom of the column.
Advantages
- Achieves 99% removal of VOCs
- The most economical alternative for flows above 50 GPM
- Most cost-effective overall
- Capable of achieving very high removal rates at very low pressure drops
- Can operate at a wide range of airflow rates
- Lower air to water ratio necessary
- Must be used for water that tends to foam
Disadvantages
- Treatment of the exiting VOC laden air needed to prevent air pollution
- Bed packing must be removed and washed or cleansed with acid
- Fouling can occur quickly
- Often need a very tall column to achieve desired efficiency, resulting in high construction costs
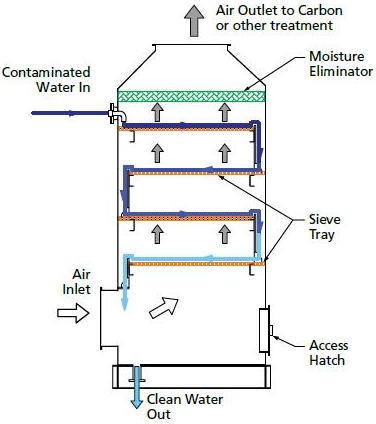
Sieve-Tray Air Strippers
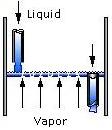
Water enters from the top of the sieve-tray air stripper and trickles down over multiple perforated trays over a weir, progressing from tray to tray. Air is bubbled up through the holes in the trays and VOCs are transferred to the vapor phase for removal. The treated water exits out the bottom after traveling across the last tray.
Equipment Design
Air stripping columns contain either trays or packing, depending on the application.
Tray types include sieve, valve, and bubble cap. The geometry of the trays affects the extent and type of contact between the vapor and liquid streams. Downcomers channel the liquid flowing from one tray down to the tray below.
Advantages
- VOC removals in excess of 99.99% are possible with sieve-tray air stripping
- Can be easily disassembled for cleaning
- Easy to prevent fouling
- Additional sieve trays may be added if greater treatment is desired
- More compact for tight areas
Disadvantages
- Treatment of the exiting VOC laden air needed to prevent air pollution
- Fouling at some point is still inevitable
- Narrow range of airflow rates to achieve volatilization without interrupting water flow
- Geometry and tray placement must be specific, often designed by the manufacturer
(Copyright Monroe Environmental Corporation, Monroe, MI)
Diffused-Aeration Tanks used for Air Stripping
Unlike the packed-column and sieve-tray air strippers, in which the water flows through or around a solid as it travels through the column, resulting in increased water-air contact, in diffused-aeration tanks the air travels directly through the water as it is bubbled into the tank. Water enters at the top of the aeration column and is allowed to pool within the tank. Air is diffused through the bottom of the tank and bubbled through the water, capturing VOCs before exiting at the top. This design is most effective when there is a greater vessel depth and smaller air bubbles passing through.
Usage Examples
One common issue that can be remediated by an air stripper is water contaminated by petroleum leaks and spills. Air strippers can easily remove benzene, ethylbenzene, toluene, xylene, and trichloroethylene. Alcohols, aldehydes, or ketones are not as easily removed by air strippers.
In the system below on the left, air is used to remove benzene derivatives, chloroethylenes, and ammonia from contaminated groundwater. On the right is an air stripping column used to remove VOCs at a potable water treatment plant.
(Copyright Monroe Environmental Corporation, Monroe, MI)
The air stripping columns pictured below are used to remove VOCs and dissolved gases from an incoming water feedstream.

The air strippers pictured below are used in a groundwater treatment plant. These units purify the incoming water through the use of trays rather than packing.

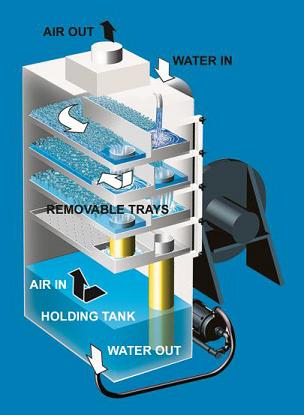
(Copyright QED Environmental Systems, Dexter, MI)
Advantages
- Simple to use
- Most resistant to fouling
- Can handle high amount of suspended solids or turbidity
- Lowest upkeep costs
- Easy to clean, simply purge water in column
Disadvantages
- Treatment of the exiting VOC laden air needed to prevent air pollution
- Not as efficient as other methods
- Cannot remove as many VOCs as sieve-tray or packed column air strippers
- Limited surface area of the water in contact with air bubbled through
Steam Stripping Columns
General Information
Steam strippers are similar to air strippers with one exception: instead of air, they use steam to strip volatile components from a liquid stream.
Steam strippers allow for the removal of heavy soluble organic compounds that air strippers cannot remove. The temperature of these columns is usually very close to the boiling point of water.
In a typical steam stripping column, the liquid feed is introduced at the top of the column and the steam input is located at the bottom. When the liquid enters the column, it flows down the column and through the packing countercurrent to the steam. The packing enhances the stripping by increasing the amount of contact between the two streams. After leaving the column, the vapor is either distributed into the atmosphere or is collected for purification.
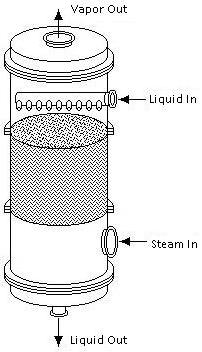
Equipment Design
Steam stripping columns contain either trays or packing, depending on the application. These trays and packing are the same as those used in distillation and absorption columns.
Tray types include sieve, valve, and bubble cap. The geometry of the trays affects the extent and type of contact between the vapor and liquid streams. Downcomers channel the liquid flowing from one tray down to the tray below.
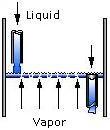
Packing types include dumped packing and stacked packing . The packing channels the liquid flowing from the top to the bottom of the column.
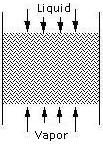
The operating pressure of a steam stripper can influence the efficiency and reliability of the column. At lower operating pressures, greater volatility and lower operating temperatures are achieved. Stream strippers operating a vacuum can be expensive but highly efficient; they allow the use of plastic internals to fight the effects of corrosive systems. Operating the column at or near atmospheric pressure results in higher volatilities and lower operating temperatures without the extra expense of a vacuum system. At higher operating pressures, the solubility of the solute increases and the separation becomes more difficult to perform. Also, at higher pressures, more steam input to the column is required for the same separation to occur.
Preheating the feed before it enters the column is important because it reduces the amount of heat added to the column to achieve the required separation. The feed is preheated by either a heat recovery exchanger that cross exchanges the feed without the effluent coming off of the bottoms, a steam heat exchanger that cross exchanges the feed with a steam stream, or a combination of both.
Usage Examples
Steam stripping columns are most widely used in the recovery of solvents and solutes, and also in the removal of Volatile Organic Compounds (VOCs) from water.
Steam stripping for wastewater purification is a distillation process where over 99% of VOCs are removed from water. Some typical VOCs are benzene, toluene, xylenes, ethylbenzene, styrene, and chlorinated hydrocarbons.
The wastewater feedstream is pre-heated and put in contact with steam in a packed or tray column. Trays or packing units facilitate contact between the contaminated water and the steam stream. The combined effects of the steam and heat cause the organic materials to transfer from the liquid into the vapor phase. As contacting proceeds down the column, the vapor becomes rich in organic materials while the wastewater gets cleaner. The organic vapor leaves the column at the top and is condensed and recovered. These hydrocarbons can be recycled back into the stripper or treated with an incinerator. The purified water that leaves the bottom of the column has very low organic contaminant concentrations and it can be recycled back into the plant’s water system.
Advantages
- Capable of achieving very high removal rates and low effluent concentrations.
- Most economical removal technique at feed concentrations above 0.1% weight organics.
- Can be made very energy efficient with heat recovery.
- Minimizes air emissions and reduces loads to incineration.
- Easy solute recovery.
Disadvantages
- Not useful to strip phenol, glycols, ethylene, propylene, glycerine, ionic compounds such as sulfonated organics, inorganic compounds (except in free gaseous dissolved form), and low volatility, highly water-soluble compounds such as acetic acid.
- Fouling.
Acknowledgements
- Chemical Engineering, Access Intelligence, LLC
- Ducon Technologies, Inc., Farmingdale, NY
- Monroe Environmental Corporation, Monroe, MI
- Layne Christensen Company, Mission Woods, KS
- QED Environmental Systems, Dexter, MI
- Sigma-Aldrich Co. LLC, St. Louis, MO
- Sulzer Chemtech Ltd., Switzerland
- Vendome Copper and Brass Works, Inc., Louisville, KY
References
- Ellerbe, R.W. “Steam Distillation/Stripping.” Handbook of Separation Techniques for Chemical Engineers. 2nd ed. Ed. Philip A. Schweitzer. New York: McGraw-Hill, Inc., 1988. Print.
- Kohl, Arthur L. “Absorption and Stripping.” Handbook of Separation Process Technology. Ed. Ronald W. Rousseau. New York: John Wiley & Sons 1987: 340-344, 399-400. Print.
- McCabe, Warren L., Julian C. Smith, and Peter Harriott. Unit Operations of Chemical Engineering. 5th ed. McGraw-Hill, New York, 1993. Print.
- Mead, E., and J. Liebbert. “ A COMPARISON OF PACKED-COLUMN AND LOW-PROFILE SIEVE TRAY AIR STRIPPERS” . U.S. Army Corps of Engineers, 1998
- Norman P. Lieberman; Elizabeth T. Lieberman: Working Guide to Process Equipment, Fourth Edition. Wastewater Strippers, Chapter (McGraw-Hill Professional, 2014), AccessEngineering
- Perry, Robert H., and Don W. Green. Perry’s Chemical Engineers’ Handbook. 7th ed. New York: McGraw Hill 1997: 14-4 – 14-10, 14-23 – 14-26, 14-38 – 14-40. Print.
- Zygula, Timothy M. “Designing Steam Stripping Columns for Wastewater.” Chemical Engineering May 2008: 52-56. Print.
Developers
- Brent Ladd
- Matthew Robertson
- Abigail Nalbandian
- Kelsey Kaplan
- Steve Cotton
- Emma TerBeek


