Analyzers are used to obtain gas samples and determine their composition. In-situ analyzers perform measurements right in the gas stream, while extractive analyzers remove a gas sample from the stream prior to analysis.
In-Situ
In-situ analyzers have sensors to take measurements directly in the gas stream. This allows for a reading without any time delay.
General Information
In-situ analyzers contain infrared, ultraviolet, or electrochemical sensors. Some sensors are housed in probes placed right in the gas stream. Transmitter/receiver units that sit across the pipe can also be used.
Equipment Design
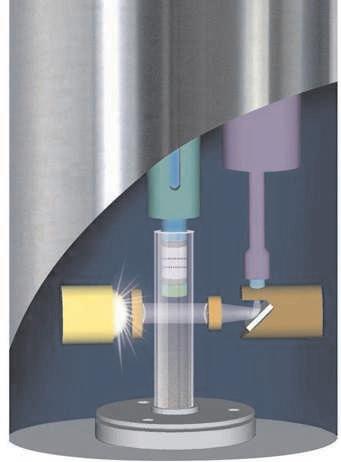
This schematic shows a cross-section of an in-situ analyzer. The analyzer probe sits directly in the gas stream, and the probe sensors detect the concentration of the species of interest. For this particular in-situ analyzer, the concentration of the species is measured by using a spectrometer and a xenon flashlight beam.
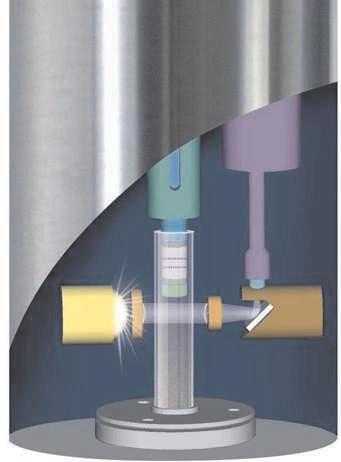
The analyzer units can be configured to relay this information to a variety of devices, such as computers, strip charts, or modems. A purge air unit is included to keep the sensors from overheating and prevent contamination.
Usage Examples
In-situ analyzers are used in a variety of applications, including the steel and paper industries.
The power plant shown here uses in-situ analyzers to analyze emissions. Specifically, sulfur and nitrogen dioxide levels are monitored.
Shown to the right is an in-situ oxygen analyzer used to analyze flue gas streams. Flue gas streams are the gaseous products of combustion from boiler furnaces or similar equipment. They consist chiefly of carbon monoxide, carbon dioxide, oxygen, nitrogen, and water vapor. The analysis of the flue gas is used to determine the efficiency of the furnace.
(Courtesy of Emerson Process Management, Chanhassen, MN)
Advantages
- Small.
- Direct measurements reduce time and give greater accuracy.
- Can be used with a variety of pipe sizes and flow rates.
Disadvantages
- Analyzers need to be calibrated.
- Probes and sensors must be designed to function at gas stream temperature and pressure.
Extractive
In extractive measurement, a gas sample is taken from the stream, prepared, and then evaluated. This method allows for a wide variety of applications.
General Information
The composition of the gas stream sample is determined using one of three sensors: infrared, ultraviolet, or electrochemical. Analyzers must be calibrated to a specific range for each application.
Equipment Design
A sample unit is attached to the pipe that carries the gas to be analyzed. The sample unit takes a sample of the stream and sends it to the analyzing unit, where it is analyzed. After the analysis, the sample gas can be recycled or purged to the atmosphere.
Usage Examples
Extractive analyzers are used in various industries, such as in glass and petrochemical manufacturing. They could be used to monitor emissions in cement manufacturing plants or to monitor oxygen concentrations in ethylene oxide plants to prevent explosions.
Advantages
- Can detect extremely low concentrations.
- Many applications possible.
- Can handle a variety of flow rates and pipe sizes.
Disadvantages
- Larger than in-situ analyzers.
- Delayed analysis of gas because it must pass from stream to sample unit to analyzing unit.
- Analyzers must be calibrated to specific ranges.
Acknowledgements
- Emerson Process Management, Chanhassen, MN
- Endress+Hauser, Inc., Greenwood, IN
References
- Perry, Robert H. and Don W. Green. Perry’s Chemical Engineers’ Handbook. 7th ed. New York: McGraw-Hill, 1997: 25-5; 25-8; 25-22 – 25-57.
Developers
- Chris Seadeek
- Henry Chen