Petroleum conversion is the final step in the petroleum refining process. Petroleum conversion can be broken down into three separate chemical processes: Decomposition, Alteration, and Unification. Decomposition processes involve the use of thermal or catalytic methods to crack large hydrocarbon molecules into smaller, more valuable hydrocarbons. Alteration processes are used to make small changes to hydrocarbon molecules, resulting in a product with more desirable and valuable properties, such as a higher-octane number. The Unification process uses chemical reactions to combine smaller liquid and gaseous hydrocarbons into medium length molecules. By combining these processes in various configurations, refineries are able to produce a consistent product from a wide range of raw material compositions.

Decomposition

Alteration

Unification
Petroleum Refining Process Map
Decomposition
Decomposition units are used to process and chemically alter the heaviest molecules that emerge from the initial distillation column in the fractionation step, producing more valuable products. Depending on its composition, the decomposition product can either be mixed directly with the refinery’s bulk gasoline product or be processed further in other refinery units before mixing with the gasoline product.
Coking and catalytic cracking units are commonly used for decomposition reactions with the lowest quality petroleum feed. Using a combination of heat and pressure, coking units “crack” or break the bonds of larger hydrocarbon molecules, converting them into smaller hydrocarbon molecules and generating coke, a carbon residue. The cracked hydrocarbons are passed to a fractionator where they are further purified based on molecular weight. Catalytic cracking units, on the other hand, upgrade the quality of their liquid feed directly, producing a mixture that contains performance-enhancing branched and aromatic hydrocarbons. In addition to feed coming directly from the primary distillation column, catalytic cracking units will often process the heavier molecules emerging from a coking unit.
Coking
There are two types of coking units: delayed and continuous. Delayed coking is a semi-continuous process in which multiple reactor drums are operated in batch mode at high temperatures and pressures, producing the cracked fuel products as well as coke, which is deposited on the reactor walls. The product is sent to a fractionator to be further purified, and the coke is removed from the drums using hydraulic cutters.
Continuous coking units require two separate units. In the first unit, steam is pumped through the base of the reactor, fluidizing a bed of fine coke particles. The hot fluidized particles mix with the hydrocarbon feed, causing the hydrocarbons to decompose, creating lighter hydrocarbons and coke as a by-product. The cracked products leave through the top of the reactor and are sent to a fractionator. Excess coke from the reaction becomes mixed with the fluidized coke. To prevent an accumulation of material, the coke is continually circulated to a second unit, where it is separated by particle size into fractions. The smaller particle fraction is reheated and sent back to the reactor unit, while the larger particle fraction is removed from the unit to maintain steady state operation.
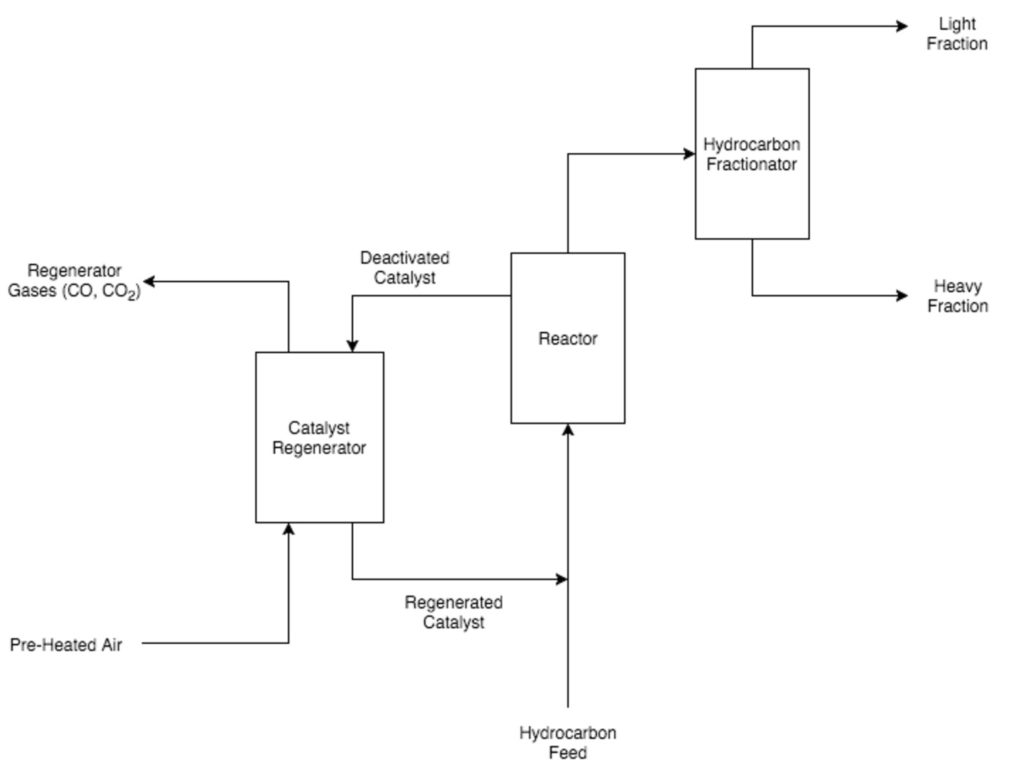
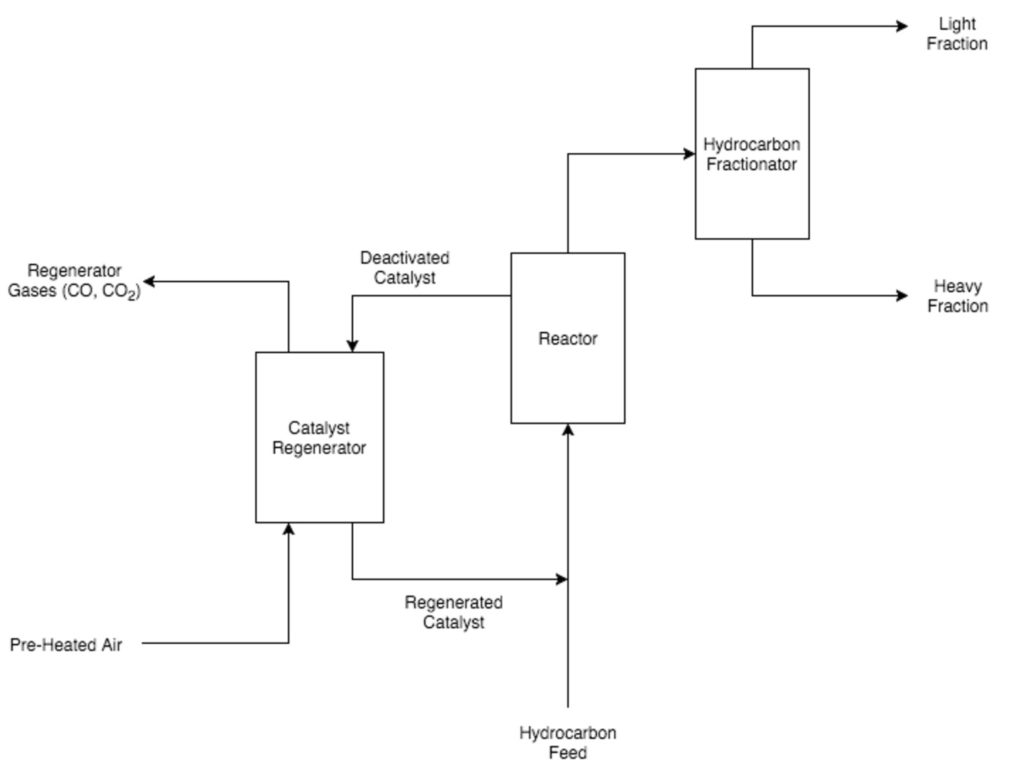
Catalytic cracking
Three types of catalytic cracking processes exist fixed-bed, moving-bed, and fluid-bed. Today, fluid-bed, otherwise known as fluidized catalytic cracking (FCC), is the most commonly used catalytic cracking method. FCC units contain zeolite, a clay-like alumina-silica catalyst that provides convenient cracking sites for the hydrocarbon molecules. Furthermore, because of the small size of individual zeolite particles, it behaves like a fluid when it is pumped through pipes or a reactor. Coke build-up results in decreased catalytic activity over time.
To eliminate the layer of coke and regenerate the catalyst, the coke-covered zeolite is burned in the presence of air, forming carbon monoxide and carbon dioxide, which are easily removed from the catalyst-regenerating unit. After being burned and regenerated, the zeolite particles are mixed with the hydrocarbon feed entering the reactor unit. An added bonus to this mixing step is that the zeolite particles effectively transfer heat from the regenerator unit to the hydrocarbon feed, which helps vaporize the hydrocarbon feed and eliminates costs for pre-heating.
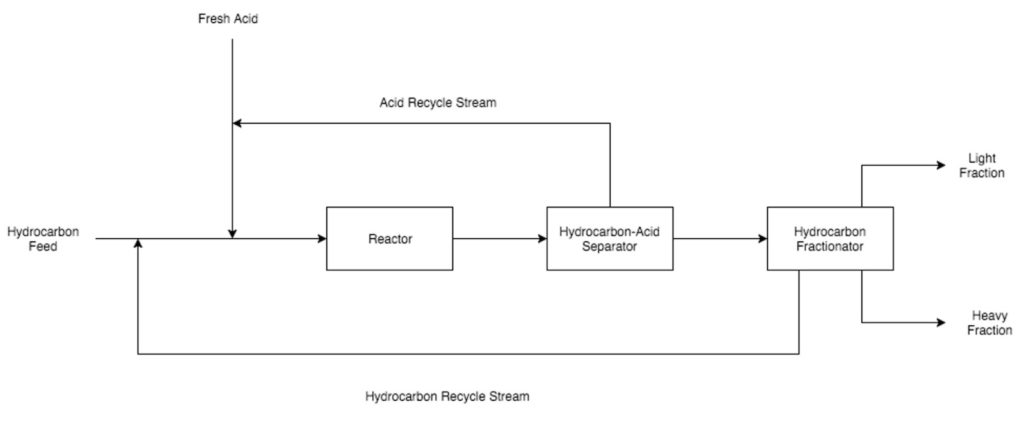
Purification
Coking and FCC reactor units use a cyclone to purify their products. In continuous coking units, the solid coke is sent to a solids separator to either be removed from the reaction unit or reheated and sent back into the reaction. In FCC units, the catalyst is sent to the regeneration unit. Cracked products from delayed coking units are passed straight to a distillation column because the hydrocarbon vapor does not contain any solid material.

After passing through the cyclone separator, the hydrocarbon vapors enter a distillation column, where the hydrocarbon vapors are separated into heavier and lighter fractions. The heaviest fractions are recycled and sent back to the reactor feed to go through the unit for a second pass. The lightest fractions are either blended with the refinery’s final gasoline product or processed further in other refinery units.
Alteration
Alteration processes are used to upgrade the octane number of products obtained throughout the refining process. A higher-octane number means that the fuel can withstand a greater pressure before detonation, increasing the performance of the fuel in high-pressure environments such as jet engines or internal combustion engines.
Reforming and isomerization are two common alteration processes. Both processes use catalysts to carry out the reaction. Catalytic reforming can be used with a wide variety of feeds, but isomerization is usually limited to butane, pentane, and hexane feeds. Reforming and isomerization follow similar paths through their reaction units. The feed is first preheated in a furnace and then passed through a catalytic reactor to form the desired products. Finally, the products are passed through separators and distillation columns to separate the desired hydrocarbon products from the waste.
Preheating the feed
Direct-fired furnaces are used to preheat a mixture of hydrocarbon feed and hydrogen up to high temperatures, ranging from 450°C to 520°C. The hydrocarbon feed usually consists of what is known as “straight-run gasoline”, meaning that it comes straight from the refinery’s distillation column. The hydrocarbon feed is sometimes treated to remove components such as sulfur or nitrogen that could poison the reaction catalyst. Direct-fired furnaces can also be inserted between individual reactors to reheat the reactants and make up for the heat lost due to the highly endothermic nature of the reforming reaction.

Production
Three types of reactors are used in catalytic reforming: Fixed-bed, fluidized-bed, and moving-bed reactors. Temperatures range from 400°C to 900°C, and pressures can range from 200 to 1000 psi. Despite the use of different catalysts and reactor conditions, all three reactors can effectively carry out the reformation process. Based on the composition of the feed, four types of reactions will occur throughout the process. Dehydrogenation results in the formation of aromatic structures where there were previously hydrocarbon rings, producing hydrogen gas as a by-product. Dehydrocyclization converts straight chain hydrocarbon molecules into ring-shaped aromatic hydrocarbons, also producing hydrogen gas a byproduct. Isomerization converts straight chain hydrocarbon molecules into branched or iso-hydrocarbon molecules such as iso-octane. Finally, hydrocracking breaks apart the largest hydrocarbon molecules into smaller molecules, which is comparable to the reaction that takes place during thermal and catalytic cracking.
The catalyst used in reforming reactions will vary based on the composition of the feed, pretreatment of the feed, and the reactor type. For example, platinum catalysts cannot be used with feeds that have not been pretreated to remove sulfur, as the sulfur will poison the catalyst, leading to highly reduced yields and increased costs to replace the poisoned catalyst. Due to the high reaction temperatures, layers of coke (pure carbon) will build upon the catalyst, gradually leading to deactivation because of reduced surface area. To regenerate the catalyst, it is reacted in the presence of oxygen to remove the coke layer.
Isomerization reactions also use a catalyst. Commonly used catalysts include platinum or aluminum chloride with anhydrous hydrochloric acid. The catalytic reactions that take place in isomerization are more selective than reforming reactions. Molecules are only being rearranged; they are not being cracked or dehydrogenated.

Fixed-bed reactors are used for a reforming process known as continuous catalytic regeneration, or CCR. Utilizing a reactor known as a swing reactor, CCR allows refineries to keep multiple reactors on-stream while other reactors are swung off-stream for catalyst regeneration. Reactor conditions are at high temperature (450°C to 550°C) and pressure (200 to 1000 psi). The conditions can vary based on the catalyst and feed composition. Platinum is the most common catalyst utilized in CCR units. In addition to a high yield of reformate, the CCR process also provides a constant supply of hydrogen, due to its continuous operation.
In a fluidized-bed reactor configuration, a separate reactor is integrated with the main reforming reactor, allowing for continuous regeneration of the catalyst. Molybdena (Mo2O3) catalyst is used for this reaction. Reactor conditions can range from 480°C to 950°C and 200 to 300 psi.
In moving-bed reactors, a catalyst consisting of Cobalt-Molybdate pellets flows down the reactor under the influence of gravity. The feed, which consists of hydrocarbon vapor and hydrogen gas, moves upward against the flow of catalyst. Reactor conditions can range from 425°C to 480°C and around 400 psi.
Isomerization reactions are carried out in fixed bed reactors, with temperatures ranging from 40°C to 480°C and pressures ranging from 150 to 1000 psi. Butane isomerization often takes place in the lower end of the temperature range while pentane and hexane isomerization take place in the higher end.
Purification
Hydrogen is separated from the hydrocarbon products after the mixture has passed through all of the reactors. After separation, the hydrogen gas is split into two streams – one hydrogen stream is recycled back into the reforming unit feed, and the second hydrogen stream is piped to other units in the refinery where it can be utilized for other processes, such as chemical manufacturing or energy production.
The hydrocarbon products are separated in a distillation column placed at the end of the reforming unit. The two major products are reformate and light petroleum gases (LPGs). Reformate is removed through the bottom of the distillation column and sent to storage, where it can be blended with other gasoline components to produce a valuable high-octane product. LPGs, such as butane and propane, are removed from the top of the distillation column and sent to other processing units in the refinery.
After passing through the isomerization reaction unit, the products go through a purification process similar to a reforming unit’s purification process. First, hydrogen is separated from the hydrocarbons. Any unreacted hydrogen is recycled back into the isomerization unit feed. After the hydrogen separation, the hydrocarbons are passed through a distillation column. Iso-hydrocarbons such as isobutane are removed from the bottom of the column and sent to storage. Unreacted hydrocarbon feed is removed from the top of the column and recycled back to the start of the isomerization unit to be combined with the original feed.

Unification
The lightest molecular weight products from other units in the refinery are further processed through unification. Both alkylation and polymerization are unification processes that use a combination of heat, pressure, and catalysts to react and combine hydrocarbon molecules that contain double bonds. In an alkylation unit, light petroleum gases (LPGs) containing double bonds are reacted with isobutane in the presence of a catalyst to produce alkylate, a high-performance fuel containing larger iso-hydrocarbons, such as iso-octane or iso-nonane.
Polymerization units operate in a similar manner, except polymerization reactions do not have an isobutane component. Inside the reactor, LPGs are reacted in the presence of a catalyst to form larger hydrocarbon molecules. These heavier molecules are collectively known as polymer gasoline. Polymer gasoline has physical properties similar to other gasoline components, meaning it can be blended with gasoline produced from units throughout the refinery.
After both alkylation and polymerization reactions are carried out, the products are passed through various separators to isolate the unreacted LPGs, waste materials, and the desired products.
Production
Catalysts are used in alkylation units to help the reactions proceed at a lower temperature, decreasing the possibility of polymerization of the LPG reactants and favoring the alkylation reaction between LPGs and isobutane.
Hydrofluoric acid and sulfuric acid are two catalysts that are commonly employed in alkylation units. Hydrofluoric acid can be used for higher temperature reactions and all feed compositions. Sulfuric acid is used for lower temperature reactions but due to an unfavorable side reaction, it cannot be used with LPG feeds that contain ethylene gas.
The hydrocarbon feed to alkylation units consists of two components: Isobutane and LPGs that contain double bonds. The isobutane feed often comes from the isomerization reaction unit, while the LPG feed originates from either the initial distillation column or the cracking units. Conditions in the reactor will vary based on which catalyst is being used and the composition of the LPGs. A hydrofluoric acid-catalyzed reaction can be run at a higher temperature because the hydrocarbons are less likely to form polymers, which is an undesirable side reaction for the alkylation process.
Polymerization units also employ a catalytic acid. Rather than using sulfuric or hydrofluoric acid, polymerization units use phosphoric acid. Combinations of phosphoric acid and solid pellets can be used to carry out specific reactions, but as a general rule, most catalytic polymerization units use liquid phosphoric acid.
Unlike the alkylation unit, the polymerization unit’s hydrocarbon feed consists solely of LPGs that are obtained from either the initial distillation column or the cracking units. Hydrocarbon feed is combined with the acid catalyst inside the reactor. The temperature inside the reactor needs to be closely monitored in a polymerization unit for two reasons. First, proper reaction conditions need to be maintained to ensure that the product being formed is limited to dimers, trimers, or tetramers (reacting two, three, or four individual molecules of LPG). Second, the polymerization reaction is highly exothermic, meaning the reaction generates a large amount of heat. The reaction could quickly result in a major safety issue or runaway reaction if the heat being released isn’t properly managed.
Purification
After the hydrocarbon-catalytic acid mixture has passed through the reactor, it enters a liquid settler, where it will gradually separate out into a hydrocarbon-rich phase on top and an acid-rich phase on the bottom. The acid-rich phase is recycled back into the alkylation unit feed to be reused as a catalyst. The hydrocarbon-rich phase is sent to a distillation column.
Alkylate, the desired product, is removed through the bottom of the distillation column, while various by-products and unreacted material are removed through the top section of the column. After distillation, alkylate is sent to another unit in the refinery where it is blended with fuel products from other units to create the final gasoline mixture. This mixture is sent to storage and eventually sold. Unreacted material and by-products are first sent to a stripper to ensure that there is little to no remaining acid catalyst present in the gas. After stripping, the unreacted materials and by-products are passed through a second fractionator. Unreacted materials such as isobutane are recycled back to the feed entering the alkylation unit. By-products such as propane and butane are sent to other units in the refinery to be treated and stored.
In a polymerization unit, the hydrocarbon-catalytic acid mixture undergoes a purification process similar to that of the alkylation unit. Taking advantage of liquid phase equilibrium properties, the hydrocarbons are separated from the acid catalyst using a liquid settler. The acid is recycled and sent back to the entrance of the reactor to be recombined with the reaction feed. Flash drums or distillation columns are used to further separate the hydrocarbon mixture into polymer gasoline, unreacted LPGs, and by-products such as propane and butane. The light petroleum gases are recycled and sent back to the entrance of the reactor, while by-products and polymer gasoline are sent to storage.
Acknowledgments
- Alteration – Monroe Environmental Corporation , Monroe, MI
- Decomposition – Sierra Monitor Corporation , Milpitas, CA
- Falmouth Products Inc. , Falmouth, MA
- Mac Process Inc. , Kansas City, MO
- Nutec Bickley , Santa Catarina, Mexico
- Sulzer Chemtech Ltd. , Switzerland
- Unification – ROBATEL, Inc. , Pittsfield, MA
References
- “Petroleum Refining Process.” OSHA Technical Manual. Section IV: Chapter 2. United States Department of Labor, Occupational Safety & Health Administration, n.d. Web. 13 Mar. 2016. https://www.osha.gov/dts/osta/otm/otm_iv/otm_iv_2.html
- Speight, James G. The Chemistry and Technology of Petroleum. 4th ed. Boca Raton: CRC Press, 2007. Print.
Developers
- Jackson Irwin




