A combustion reaction is a very fast oxidation reaction in which one species donates electrons to another. Combustion releases a considerable amount of heat and light in the form of fire and increases the number of moles of gas in a closed system, raising the system pressure. Such a pressure increase may lead to an explosion. This module will focus primarily on fires and explosions caused by combustion reactions, though many principles discussed apply to other types of explosions, such as those caused by sudden impact or shock. For more information on preventing explosions, see Pressure Control and Relief.
The most effective way to prevent a fire or explosion is to recognize the factors that cause them and know how these factors are controlled in industry. Explosions are categorized as either detonations or deflagrations based on whether they propagate above or below the speed of sound, respectively. Detonations are typically more destructive than deflagrations in that they propagate shock waves with higher pressure in a shorter duration.
Fire Risk Factors and How to Control Them
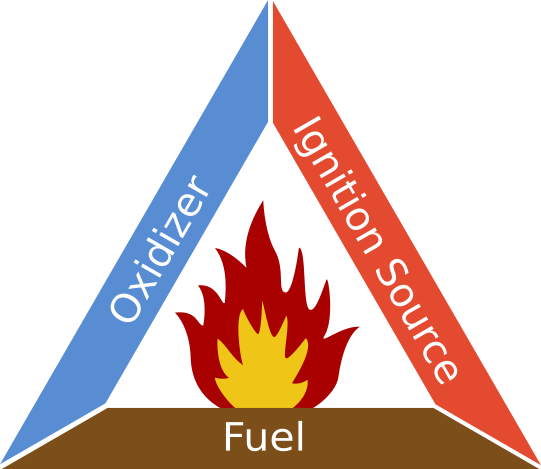
Shown below is the fire triangle, a symbol used in industry to depict fire risk factors. The sides of the triangle represent the three components necessary for a fire. The central region depicts the combustion reaction, a chain reaction that is self-sustaining as long as the three components are present. If any element of the fire triangle is removed, combustion will be unable to start or persist. Fire and explosion prevention methods are designed to make sure these three components do not interact.
Fuel
Fuel is the species that is oxidized during combustion. It can be found in the solid, liquid, or gas phases. For most fires, the fuel source will be organic matter, but many metals, such as magnesium, can also be fuel. A vaporized fuel can only ignite within a certain range of concentrations, defined by the lower and upper flammability limits. Flammability limits occur near the stoichiometric ratio for the combustion reaction but are also affected by factors including temperature, pressure, and the concentration of inert gases. One way to lower the concentration of vaporous fuel is through ventilation.
Ventilating to control fuel concentrations
Ventilation is the process of introducing air to a system from another source to improve air quality and prevent the formation of localized flammable atmospheres. Ventilation can be used to remove flammable fuels present as dust and gas from a system or dilute them until they are no longer flammable. Ventilation is normally only required in indoor plants, as the lack of confinement in outdoor plants prevents the buildup of flammable dust and gas.
Ventilation systems can be either local exhaust or dilution systems. Local exhaust systems remove gas from a confined space, while dilution systems are used for the room- or plant-scale ventilation.
Local Exhaust Ventilation
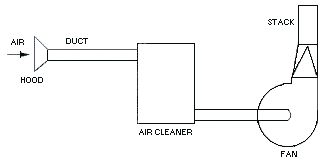
Local exhaust methods use air currents to confine fuel to a specific area and transfer it out of the plant. This is the preferred method in industry because it requires less energy and replacement gas than dilution ventilation. A local exhaust system consists of four parts: a hood to collect gas and dust, ducts to carry them from the plant to the atmosphere, fans to create the air current necessary for this process, and filters to purify the air, remove dust, and reduce pollution. It is very important to change these filters regularly to avoid a buildup of dust and other particles, which make the ventilation less efficient and present a potential fire hazard.
Fume hoods are an example of local exhaust ventilation, as shown in the schematic below. Two types of hoods are typically used to capture fumes in a local exhaust ventilation system. Slot exhaust hoods pull gases through narrow slots away from the operator through a duct in the back. Enclosed hoods are installed over an area of hazardous chemicals and pull gas through ducts away from the plant using slightly negative pressure. Enclosed hoods require less energy than slot exhaust hoods and are preferable where they can be installed.
Dilution Ventilation
Dilution ventilation lowers the concentration of fumes in a facility by pushing contaminated air out of the facility with an exhaust fan. At the same time, clean air is introduced to the facility by a makeup air duct and fan. Because dilution ventilation does not isolate workers from fumes, it should not be used in facilities where harmful vapors can be inhaled.
Oxidizer
When reaction conditions are met, oxidizers strip electrons from the fuel, resulting in combustion. Oxidizers are typically highly electronegative and can be solids, liquids, or gases. Oxygen, the most ubiquitous oxidizer, is particularly problematic in industry since it is present in reactive concentrations in Earth’s atmosphere.
Inerting to control oxygen concentration
Inerting involves replacing an oxygen-rich atmosphere in a vessel with an inert gas, typically nitrogen or argon, to reduce the oxygen concentration well below the limiting oxygen concentration (LOC). Below the LOC, the chemical chain reaction responsible for combustion becomes impossible to sustain, and no reaction will occur.
Where there is a risk of combustion, control systems are typically equipped with oxygen monitors to trigger inerting automatically whenever concentrations approach unsafe levels. Ideally, oxygen concentrations are kept at least 4% below the LOC. These oxygen levels are unsafe for breathing, so only individuals with respirators may safely enter inerted vessels.
Common inerting techniques include vacuum, pressure, sweep-through, and siphon purging.
Vacuum Purging
Vacuum purging removes as much gas from a system as possible, then refills the vessel with inert gas. Many reactors are designed to handle vacuum pressures. In especially oxygen-sensitive applications, pressure may be repeatedly cycled until oxygen concentrations on the order of parts per million are achieved. Vacuum purging is the preferred method in industry because it requires the lowest quantity of inert gas out of all inerting methods.
Pressure Purging
Pressure purging is the opposite of vacuum purging and is often used in storage vessels or other containers that cannot handle low pressures but can withstand high pressures. This process involves pressurizing a vessel with inert gas to lower the concentration of oxygen within the vessel. The vessel is then vented to system pressure, decreasing the number of moles of oxygen within the vessel. Pressure purging uses more inert gas than vacuum purging but is compatible with most vessels.
Sweep-through Purging
Sweep-through purging is the process of simultaneously removing gas from and adding inert gas to the vessel in equal quantities, which lowers the concentration of oxygen within a vessel without changing the vessel’s overall pressure. This technique is common when a vessel’s pressure tolerance has not been rated or a constant-pressure process is desired. Sweep-through purging can be done while maintaining a steady state in the vessel. However, because inert gas is simultaneously added and removed from the vessel, much more of the inert species is required. Another disadvantage to this technique is that the flow path of inert gas within the vessel must be considered to ensure there are no pockets with dangerous oxygen concentrations.
Siphon Purging
Siphon purging is a variation of sweep-through purging meant to decrease the amount of inert gas required by using liquids to remove oxygen. The vessel is filled with a liquid such as water, then drained while an inert gas is added. The liquid displaces or absorbs oxygen from the vessel, and is in turn displaced by the inert gas. If siphon purging can be used, it may prove to be significantly less expensive than traditional sweep-through purging since it greatly decreases the amount of inert gas required.
The advantages and disadvantages of the four types of purging discussed in this article are summarized below :
| Purging Method | Operation Pressure | Advantages | Disadvantages |
| Vacuum purging | 0 atm – system pressure | Cheap and requires relatively little inert gas. Reactors can often handle vacuum pressure. | Storage vessels are often not rated for low pressure. |
| Pressure purging | System Pressure | Compatible with most vessels. | Requires more inert gas than vacuum purging. |
| Sweep-through purging | System Pressure | Can be integrated into a continuous process. Does not change pressure. | Requires more inert gas than any other purging method. Localized flammable oxygen concentrations may occur. |
| Siphon purging | Liquid pressure | Cheap and requires relatively little inert gas. Does not produce a vacuum. | Vessel contents must not be reactive with purging fluid. Changes pressure, unlike sweep-through purging. |
Ignition Source
Ignition occurs when a combustible system surpasses the activation energy needed to begin reacting. An ignition source provides this energy and begins the combustion reaction. High ambient temperatures may serve as an ignition source or make substances more susceptible to ignition by other means. Flammable substances become increasingly more susceptible to begin combustion starting at the flashpoint and ending when the autoignition temperature is reached.
The flashpoint is the minimum temperature at which a substance combusts. Flash fires dissipate quickly, providing they do not ignite other flammable substances. Slightly above the flashpoint lies the flame point, the minimum temperature required for a continuous combustion reaction, also known as burning. Beyond the flame, the point is the autoignition temperature. Autoignition occurs when a substance’s temperature is high enough to trigger combustion without the aid of another ignition source. The presence of an oxidizer is a requirement regardless of temperature.
Common ignition sources include uncontrolled exothermic chemical reactions and electrostatic discharge.
Preventing Unsafe Static Buildup to Prevent Ignition
A static discharge releases enough energy to ignite many flammable materials and should be avoided in flammable environments. Static discharge occurs when enough charge accumulates in an object, causing electrons to jump into another object to equilibrate charge. A typical electrostatic discharge releases several millijoules of energy. Although this may seem like a small amount of energy, it is sufficient to cause ignition when applied to a small area.
Creating static electricity is inevitable in industry. One factor to consider is that poor electric conductors will build a static charge faster than good conductors. Whenever an electric insulator is separated from another material, such as when mixing or conveying insulating dusts and fluids, a charge will accumulate. Another risk factor to consider is induction, through which other charged objects can polarize positive and negative charges, leading to charge accumulation in different regions of an object. The charge can also be transferred if charged liquid droplets splash against an object. Lastly, the liquid will generate minute quantities of static electricity at its interface with any other phase.
Preventing static buildup is critical to preventing ignition. This can be achieved through charge relaxation, bonding and grounding, and the use of intrinsically safe and explosion-proof equipment, as described below. Should an explosion occur, explosion-proof equipment can help mitigate its damage.
Charge Relaxation
If a charged substance remains in contact with a conductive surface, its charges will safely balance out over time via a process known as relaxation. In order to allow charge relaxation to occur, plants may enforce a set holding time before interacting with insulating fluids from a pipe. Another tactic is to allow more contact time between pipe and fluid by using a larger diameter pipe. This slows down the flow rate of the fluid and increases the surface area in contact with the conductor, both of which allow for more charge relaxation.
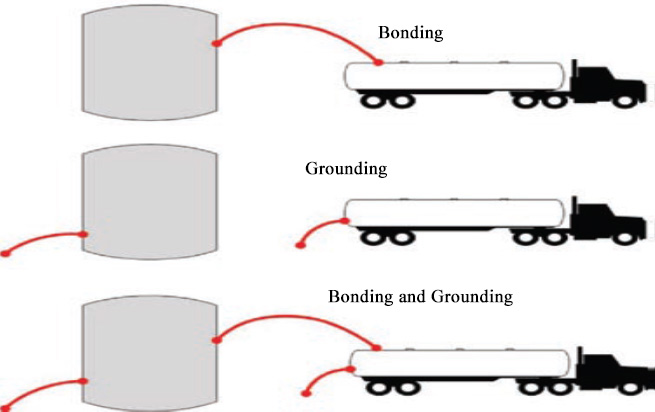
Bonding and Grounding
Bonding and grounding use the principle of charge relaxation to mitigate static buildup when transferring flammable substances between containers. Bonding connects two containers to each other with a conductive wire, balancing the charges between them. The containers are each attached to a ground, which will absorb any net accumulation of charge. When properly bonded and grounded, static charge flows to the ground as the material is transported between containers.
Intrinsically Safe and Explosion Proof Equipment
Intrinsically safe is an international standard maintained by several different authorities. Intrinsically safe equipment has been certified to limit the energy storage and power of equipment so that it cannot spark or become hot enough to combust.
Special precautions beyond intrinsic safety must be taken for electrical equipment operating in environments susceptible to combustion. The United States National Electric Code designates equipment and areas within a workplace as Explosion Proof (XP) if they meet these regulations. An area may be designated Explosion Proof if all permanently installed electrical equipment, including outlets and lighting fixtures, are explosion-proof. This certifies workers can safely plug in and use XP equipment in these locations.
Additionally, XP equipment housings are designed to contain or mitigate an explosion, should one occur. This is typically accomplished by creating paths within the device that allow gas to expand and cool. XP equipment may also be designed to not spark or to isolate electrical components from flammable gases and dust.
Fire Suppression
Various equipment and automatic systems are available to mitigate the devastating effects of a fire even after ignition.
Fire Blankets
Fire blankets can be thrown on top of small fires or wrapped around an individual to smother a fire. Smothering will deprive the fire of oxygen, causing the fire triangle to break and the fire to extinguish. Fire blankets are made of flame retardant material, often wool or Kevlar, and coated in a fire-resistant chemical. Fire blankets will typically be located along the wall of a building in a red bag, canister, or cabinet.
Fire Extinguishers
Fire extinguishers dispense a chemical designed to isolate the components of the fire triangle. Fire extinguishers should only be used by properly trained individuals when they will not cause harm to another individual and when multiple routes to escape the fire exist. Extinguishers come in multiple types which must be matched to the type of fire, otherwise, the extinguisher may be ineffective or even exacerbate the fire. The different classes of fires are described below.
| Class | Pictogram | Type of fire | Compatible extinguisher contains |
 |  | Ordinary flammable solid (nonmetals) | Water-based foam, water mist, dry chemical, halon agent |
 | 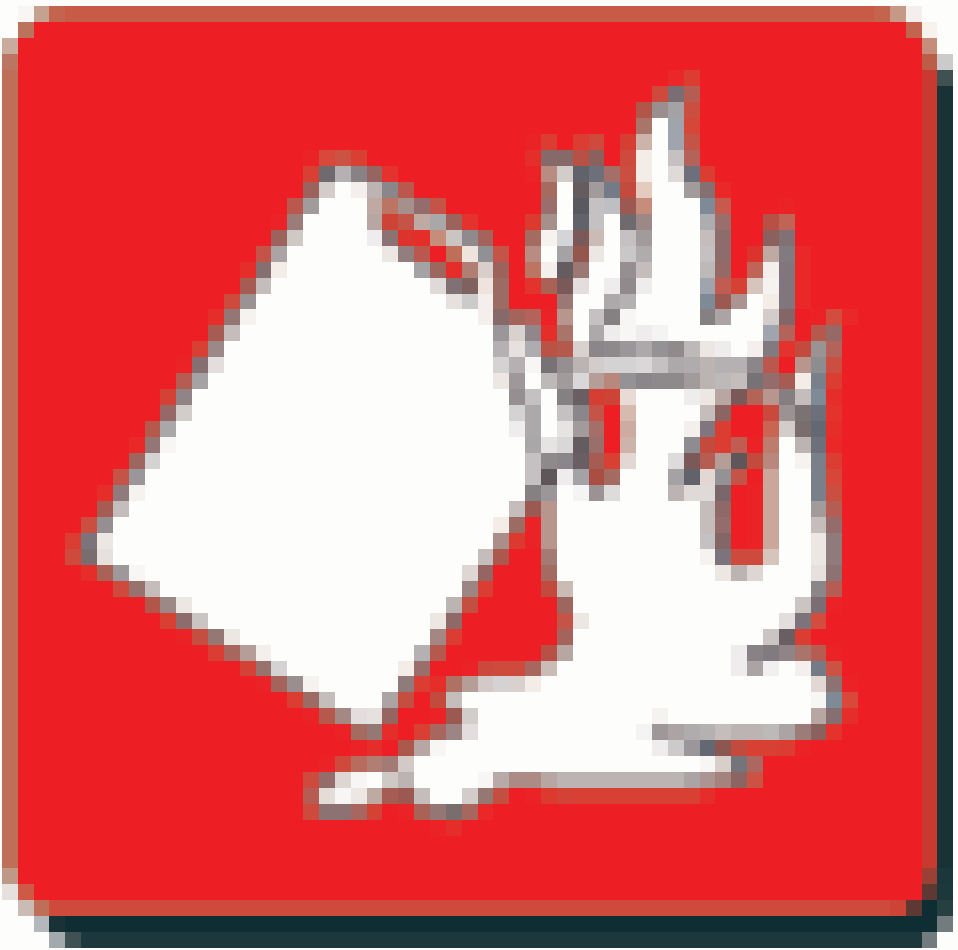 | Hydrocarbon, grease, and flammable liquid | Carbon dioxide or a dry chemical, halon agent |
 |  | Electrical Fires | Carbon dioxide or a dry chemical, halon agent |
 |  | Metal Fires | Dry powder |
 | 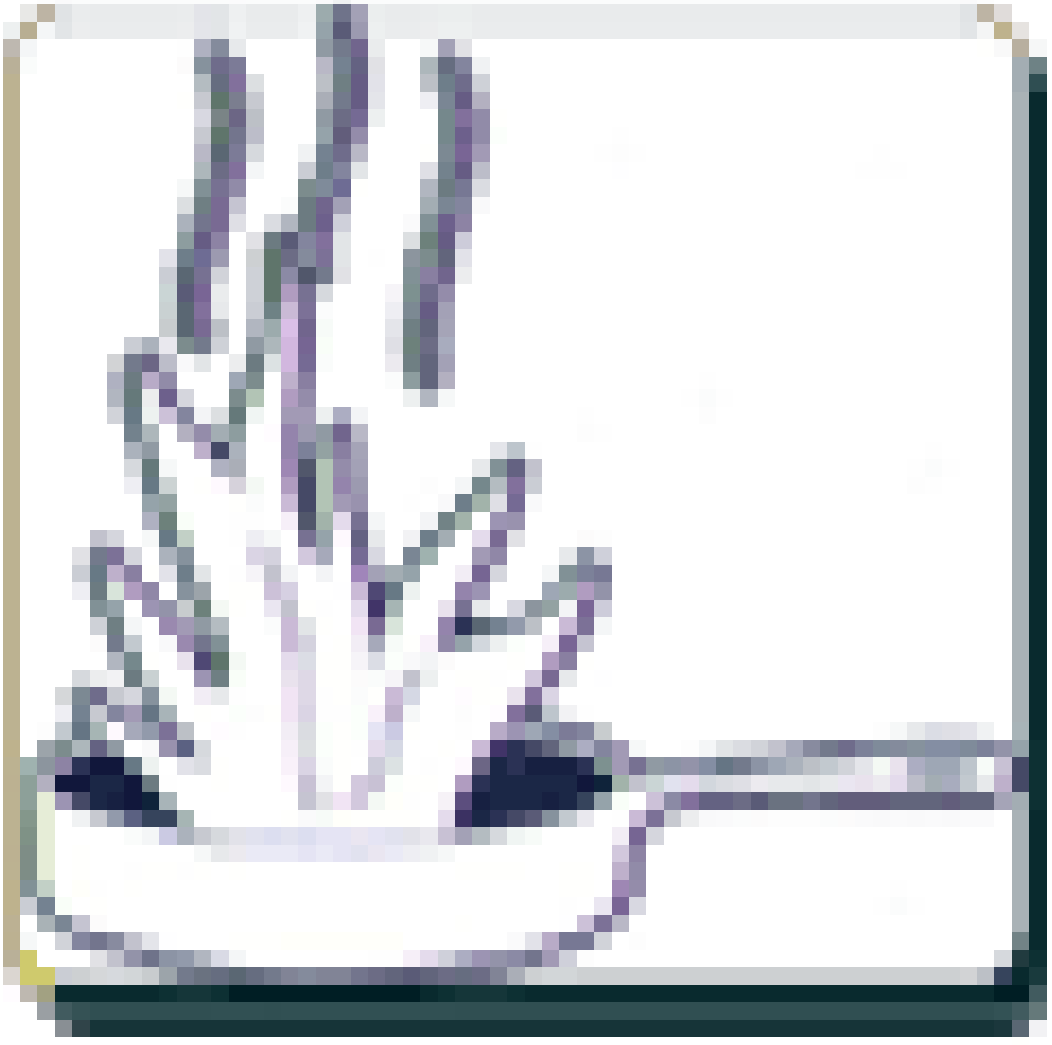 | Kitchen fires (cooking oils, hot stoves, etc.) | Wet chemical |
Fire extinguishers should not be used in the event that the class of fire cannot be readily determined or it is suspected that the fire may be of multiple classes that the extinguisher does not fully encompass. For example, a gasoline fire that ignites wood is considered both class B and A and cannot be suppressed by a class A or B extinguisher, but can be by the multi-class ABC extinguisher.
The ABC extinguisher is the most common type of fire extinguisher used today. The designation ABC means that the dry chemical agent is capable of extinguishing fires of class A, B, C, or any combination of the three. ABC dry chemical is effective against class A and B fires because it does not burn and can form a layer on the surface of materials to isolate them from oxygen. The dry chemical is also electrically insulating and can disrupt the flow of current in a class C fire in addition to the previously listed effects. However, ABC fire extinguishers also have a unique limitation: they contain a chemical that may potentially explode or form harmful gases when exposed to oxidizers other than oxygen.
Automatic Fire Extinguishing Systems
Companies may have systems installed to automatically detect fires and dispense fire extinguishing chemicals. Compared to firefighters or trained employees, automatic systems will be faster but less effective at combating a fire. The type of system implemented will differ depending on what types of fires are possible in a given area. Automatic systems are carefully considered to ensure that the class of extinguisher material is effective in types which could occur in that area.
The fire suppressing agent of an automatic system will be dispensed indiscriminately when activated, which could cause additional damage to equipment or, in the event where a fire occurs that a system is not designed to handle, make the fire worse.
Water Sprinkler Systems
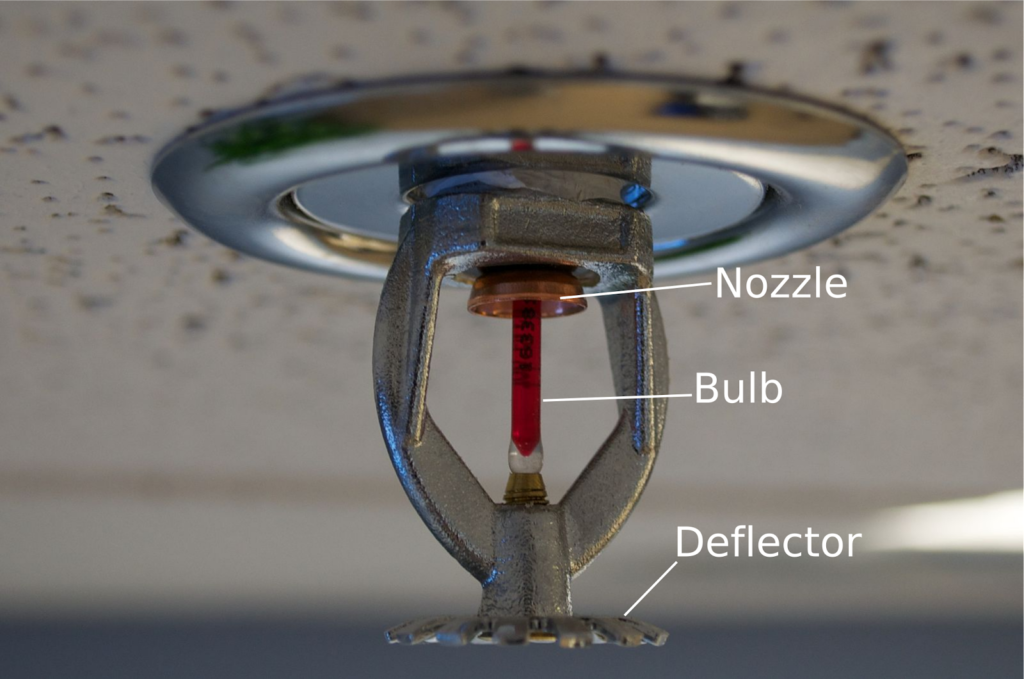
Sprinkler systems consist of numerous sprinkler heads attached to a pipe, such as the one shown below. When a fire occurs, heat causes the fluid in the bulb to expand and shatter the bulb, allowing water to flow through the nozzle. A deflector at the base of the sprinkler will divert water flow and increase the area of coverage. Each sprinkler can be activated individually because each is regulated by a different bulb. This allows for smaller, localized fires to be extinguished while minimizing the damage done by water.
Water sprinkler systems are only effective on class A fires. Though this may limit their viability where other types of fires may occur, they are among the most common and cost-effective systems to implement.
Water sprinklers exist as wet pipe or dry pipe systems:
Wet Pipe Systems
Wet pipe sprinkler systems store water in the pipe to the sprinkler is attached to. These systems are commonly used in smaller plants. When the bulb shatters, water immediately rushes out to contain a fire. In colder climates, the risk of pipes freezing should be considered if implementing this type of system.
Dry Pipe Systems
In order to combat freezing, buildings may use a dry pipe system instead. Rather than water, dry pipe systems are filled with pressurized air that blocks water flow with a dry valve. In its normal state, air pressure prevents the flow of water across the dry valve. When a fire occurs, the fluid-filled bulb breaks. This allows the gas to escape and relieve pressure on the dry valve, which allows water to rush through and suppress the fire. These systems are more expensive to install and maintain when compared to wet pipe systems. Depending on the air pressure in the dry pipe system, they may also delay the release of water for up to a minute.
Deluge Systems
Larger plants and facilities may employ a deluge sprinkler system. In this type of system, all sprinklers are connected to a common control point. Pipes are kept dry until the control system allows water to flow. Unlike the wet and dry valve systems, which are activated in response to high temperatures, deluge systems are activated by external control. This allows for more advanced detection systems, such as smoke or heat detectors.
Deluge systems are typically used in plants where a fire could be disastrous if not immediately contained. Deluge systems are more likely to contain large fires because they spray water through every nozzle when activated. This comes at the risk of causing more water damage than other systems. Furthermore, containment or disposal systems for runoff water are necessary for deluge systems to prevent flooding.
Other Automatic Systems
Automatic fire suppression systems can also use extinguishing agents other than water to better suit their needs. While water supplies are virtually unlimited, other agents require reservoirs, which can be depleted. Common alternatives include water-foam sprinklers and ABC dry powder systems.
Water-Foam Systems
Water-foam systems use a chemical to create a foam and specialized sprinklers to aerate the foam, increasing the covered surface area dramatically. Compared to sprinkler systems, water-foam systems will have increased coverage and suppressive capabilities, especially against more intense fires. These systems are, however, more expensive and require a reservoir of foaming agents. The video below shows a test of a water-foam system.
ABC Powder Systems
Automatic ABC dry powder systems share many similarities with both fire extinguishers and sprinkler systems. Dry powder systems consist of a reservoir of powder and a nitrogen-filled valve. When sufficient heat causes the valve to burst, powder rushes out and coats the room. As the name implies, ABC dry powder systems can suppress class A, B, and C fires. However, these systems are limited to the volume of the tank and maybe of limited effectiveness against a sufficiently developed fire.
Dust Explosion Risk Factors and How to Control Them
The risk of dust explosions is an important safety consideration in various chemical engineering processes. Dust explosions usually result in secondary explosions due to forces acting on excess confined dust that is stirred up from the initial explosion. Keeping all areas completely clean of dust and debris will help prevent this from happening.
Solid fuel sources are only capable of combustion at the surface, where they are directly exposed to oxidizers. Dust is especially dangerous due to its extremely high surface area to volume ratio and therefore combusts rapidly. For a given spherical volume of solid fuel, dividing it into 1,000 particles will increase its surface area by more than an order of magnitude. Because of this high surface area, dust can be compared to gases in terms of their combustive properties. Additionally, dust particles are less predictable than gases due to their non-uniform shapes, larger particle size, and the effects of gravity.
A dust’s surface area also makes transportation challenges, as static charge can build up rapidly and create a potential ignition source. Since a chemical explosion can be caused by rapid combustion, dust is a major explosion risk factor in the industry, especially where particulate organic matter is handled, such as with sawdust, coal dust, or sugar.
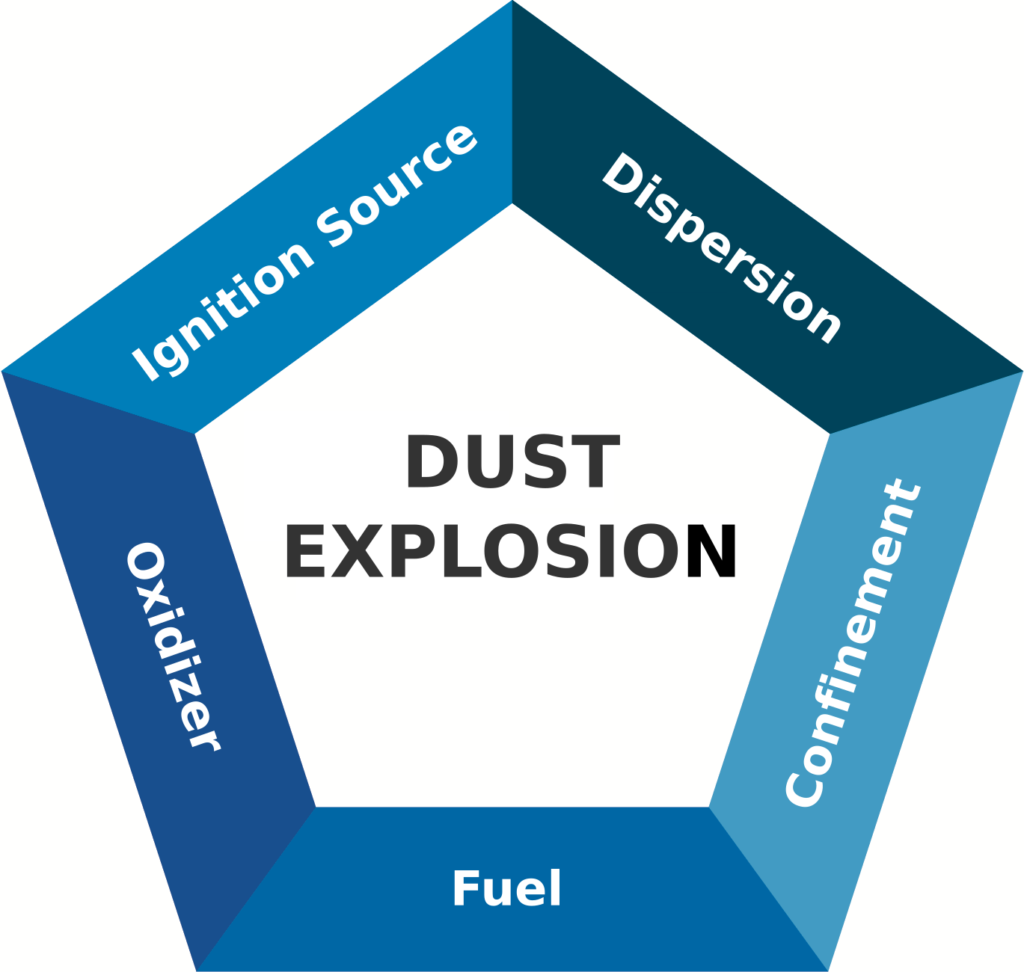
In addition to the risk factors in the fire triangle, two more elements, dispersion, and confinement, are required for dust explosions. These five elements are represented in the pentagon below. As before, removing any one side of the dust explosion pentagon will prevent a dust explosion from occurring.
Dispersion and Confinement
Dispersion is the act of distributing dust into the air, resulting in suspended dust particles. Dust burns relatively slow when confided to a surface, but can explode with high enough concentrations suspended in the air. Since these particles are very light, an air draft or a slight mechanical disturbance is capable of suspending large amounts of particles for long periods of time. In confinement, dust particles are unable to escape a system, causing their concentration in the air to increase with repeated dispersion. The minimum dust concentration for a dust explosion typically ranges from approximately 15 to 100 grams per cubic meter. These levels are easily reached through dispersion and confinement.
Proper fan ventilation and dust removal systems should be used in tandem with enclosed storage to prevent confinement and dispersion. Furthermore, dust may be able to accumulate in specific areas even when properly ventilated. If dust is to be transported within a duct, airflow must be sufficiently powerful to prevent dust from accumulating within the duct. Other safety features, such as pneumatic dust collection systems (baghouses), help reduce excess dust. Facilities that handle explosive dust should be regularly inspected and carefully cleaned.
Case Studies
Case Study 1: 2015 Tianjin Explosions
On August 12, 2015, a fire broke out in a freight container storage facility owned by Ruihai Logistics in Tianjin, China. After the fire occurred, a series of explosions followed, causing 173 deaths and costing over $1 billion in damage. The explosions began from a fire caused by the combustion of nitrocellulose, a volatile compound capable of igniting in warm air. Heated nitrocellulose in contact with air contains all the necessary elements for a fire: Fuel, oxidizer, and an ignition source.
Since several onsite chemicals were reactive with water, initial firefighting efforts were ineffective. Halon or a dry chemical should have been used to fight the fire instead of water, but firefighters were unaware of the nature of the fire when arriving on site. Improper safety measures by Ruihai were to blame for using the wrong chemical for firefighting and further exacerbating the explosion.
Once the fire had broken out, it ignited calcium carbide, releasing acetylene into the surrounding area. Acetylene entered a container of ammonium nitrate, leading to a runaway reaction and subsequent explosion in the containers. Many onsite chemicals were found to be stored above legally allowed quantities or without proper authorization. Proper storage and stricter adherence to government safety regulations could have prevented or mitigated the disaster.
Case Study 2: 2008 Imperial Sugar Dust Explosion
On February 7, 2008, an explosion occurred in a tunnel beneath a silo at an Imperial Sugar refinery in Port Wentworth, Georgia. The explosion ignited spilled sugar throughout the facility, causing secondary explosions that killed 14 workers and injured 36 more.
Prior to the incident, Imperial Sugar staff had built an enclosure around a conveyor belt to mitigate sugar spillage but neglected to include a dust collection system. Over time, airborne sugar concentrations reached hazardous levels in the enclosure. Though the exact cause is not fully understood, it is believed that an overheated bearing was the ignition source that ignited the sugar. Although sugar is typically not considered a fire risk, it can flash at temperatures above 375 °C. With the dispersed sugar dust confined within the enclosure, all five elements of a dust explosion – fuel, oxidizer, dispersion, confinement, and ignition source – were present. The explosion propagated across several buildings in the facility due to the significant quantities of spilled sugar that accumulated on various surfaces over the years.
The explosion could have been greatly mitigated or prevented by installing proper dust collection systems and implementing stricter industrial hygiene standards. This disaster prompted the Chemical Safety Board to make recommendations to OSHA regarding stricter explosive dust regulation. In 2009, OSHA began implementing these regulations.
For more case studies, please visit the CSB case studies webpage or the SAFEChE website.
Acknowledgments
- Occupational Safety and Health Administration (OSHA)
- National Fire Protection Association (NFPA)
- National Institute for Occupational Safety and Health (NIOSH)
- National Aeronautics and Space Administration (NASA)
- Chemical Safety Board (CSB)
- Federal Emergency Management Agency (FEMA)
References
- “CCPS Process Safety Glossary.” Center for Chemical Process Safety , AIChE, www.aiche.org/ccps/resources/glossary/process-safety-glossary/ .
- “Choosing and Using Fire Extinguishers.” U.S. Fire Administration , 12 Dec. 2017, www.usfa.fema.gov/prevention/outreach/extinguishers.html.
- Cielec, Lynn. “How Industrial Dust Forms and Accumulates.” Midwest Industrial Supply, 10 Apr. 2018, blog.midwestind.com/how-industrial-dust-forms-and-accumulates/.
- “Classifications of Explosives.” The Nature of Explosions, University of Turin, lem.ch.unito.it/didattica/infochimica/2008_Esplosivi/Classification.html.
- Cross, James, personal communication, 2016
- Crowl, Daniel A., and Joseph F. Louvar, Joseph F., and Daniel A. Crowl. Chemical Process
- Safety: Fundamentals with Applications, Third Edition. O’Reilly Media, Inc., 2011, learning.oreilly.com/library/view/chemical-process-safety/9780132762489/ch06.html.
- Fire Detection and Suppression Systems 3rd ed. Stillwater, OK: International Fire Service Training Association. 2005. Print.
- “Hazard Communication.” Occupational Safety and Health Administration , United States Department of Labor, www.osha.gov/Publications/OSHA3514.html.
- “Intrinsic Safety vs. Explosion Proof.” OleumTech , OleumTech, 2015, www.oleumtech.com/wp-content/uploads/2015/12/AN-1039-001-AN-OleumTech-IS-vs-XP.pdf .
- Matusewicz, Rich. “Dust Explosions: Basics and Prevention.” SACHE Training Seminar. 2008 SACHE Faculty Workshop, sache.org/workshop/2008Faculty/files/2008Workbook.pdf.
- “Static Electricity Discharge and Fire Prevention – Chemical Engineering .” Chemical Engineering Pressure Measurement Handling Difficult Process Applications , Chemical Engineering, 1 Apr. 2014,
www.chemengonline.com/static-electricity-discharge-and-fire-prevention/. - Willard, Dirk. “Move Against Static Electricity.” Operating Plants in the Chemical Industry, Chemical Processing, 6 June 2013, www.chemicalprocessing.com/articles/2013/move-against-static-electricity/.
- Zlochower, Isaac A., and Gregory M. Green. “The Limiting Oxygen Concentration and Flammability Limits of Gases and Gas Mixtures.” The National Institute of Occupational Safety and Health, CDC, July 2009, www.cdc.gov/niosh/mining/UserFiles/works/pdfs/tloca.pdf.
Developers
- John Novak
- Patrick Dillon