Pharmaceutical sprays are medicines that are administered orally or nasally through respiration. In sprays, a liquid or solid formulation is converted into a dynamic mixture dispersed in gas through atomization. An atomized spray, shown below, is able to travel into the respiratory system for local or systemic medicinal effects.
The main process steps associated with the manufacture of sprays are mixing pharmaceutical ingredients and filling the spray device with the ingredients. This module describes the production equipment used in these process steps, including the types of spray devices. The Quality Control module describes additional equipment used to monitor quality parameters during this process.
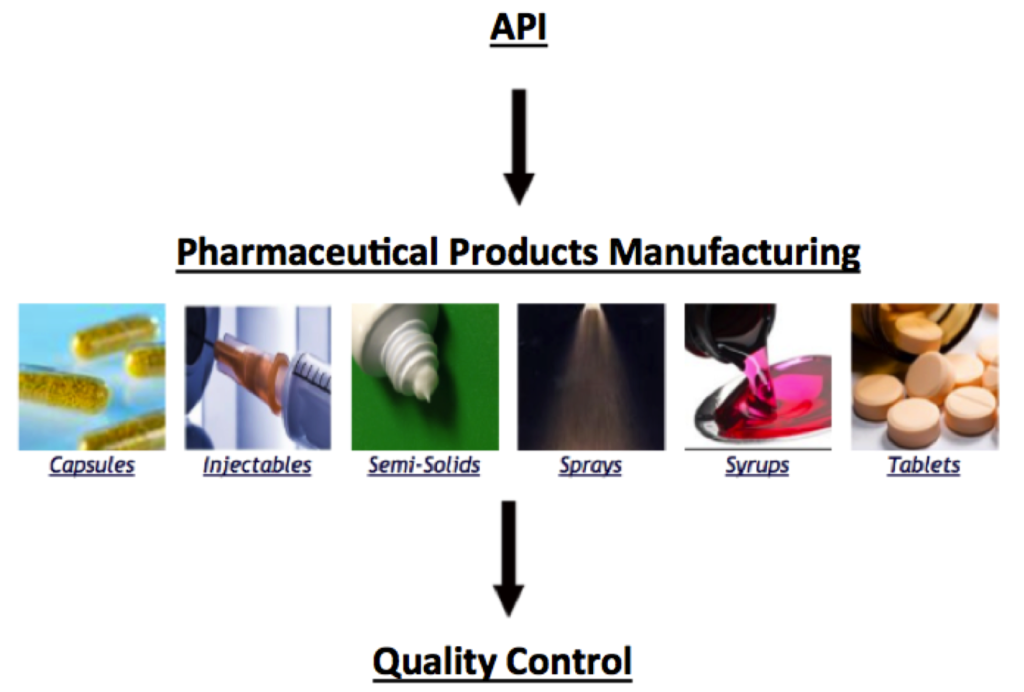
Pharmaceuticals Process Map
Mixing
Mixers accomplish the first step of pharmaceutical spray manufacturing, which is the mixing of the active pharmaceutical ingredient (API) and additional inactive ingredients or solvents into a concentrate. Several types of mixers are used in spray manufacturing depending on the process conditions and the phase of ingredients. After mixing, the process moves on to the filling step where the liquid or solid concentrate is transported into the appropriate spray device for drug administration.
Spray Filling
Once the active pharmaceutical ingredients (API) are mixed into a spray concentrate, they are pumped into the spray device that will atomize it during administration. For most spray filling processes, standard transport and storage equipment are used to handle and fill spray devices with the accurate dosage of the concentrate. Pumps, pipes, and tanks are commonly used to handle and transport the concentrates, and conveyors are used to transport spray devices to the filling location.
Aerosol Filling
For specialized spray devices, known as aerosols, the filling step requires more complicated processes and equipment. Aerosols contain pharmaceutical concentrate as well as propellant chemicals. Propellants are substances that are gases at standard temperature and atmospheric pressure. They pressurize the spray device to properly expel the medicine upon administration.
Rotary machines, such as the one shown below, can be used to fill concentrate, fill propellant, and perform any additional process steps on a rotating line of aerosol devices. These machines are used in the two processes for filling aerosols with the propellant, pressure, and cold filling.
During pressure filling, the process is held under high pressure to liquefy the propellant. The propellant is transferred under high pressure into the spray device. The entire spray bottle becomes pressurized when the propellant vaporizes, allowing proper dispensing of the pharmaceutical when the spray device is used.
Compressing pumps are used in the pressure filling process to ensure the propellant is under high pressure and in the liquid phase at the pump outlet. After pressure filling, the aerosol spray device is crimped close to prevent leakage of the propellant.
During cold filling, the process is cooled to a very low temperature to liquefy the propellant. The liquefied propellant is transferred into the spray device. At ambient temperature, the propellant vaporizes and pressurizes the spray device to the vapor pressure of the propellant. This pressurized system allows proper dispensing of the pharmaceutical when the spray device is used.
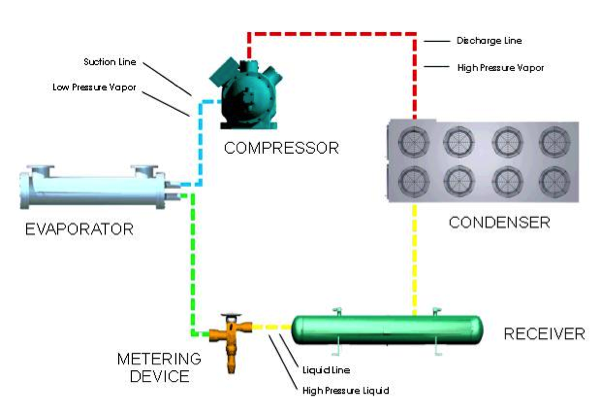
Standard transport and storage equipment are used in cold filling processes. However, the process requires refrigeration of the liquefied propellant. The refrigeration cycle for liquefying propellants is shown below, and more information on the equipment used in the cycle can be found in the refrigeration module.
Spray Devices
During spray filling, the spray devices are filled with the pharmaceutical formulation, known as the concentrate. Spray devices are specialized pieces of equipment that atomize the concentrate, which is vital to the administration of the medicine during respiration. Types of spray devices include aerosols, spray bottles, nebulizers, and dry powder inhalers.
Aerosols
Aerosols are a specialized type of spray device, which differ from standard sprays in that they contain concentrates and propellants. The propellant is a liquefied gas added to pressurize the aerosol upon vaporization. Propellants maintain suitable pressure within the aerosol container to expel the liquid concentrate when used. Hydrofluoroalkanes (HFAs) are the standard propellants used for pharmaceutical sprays and have replaced chlorofluorocarbons (CFCs), which were found to have negative effects on the Earth’s ozone layer.
A common example of pharmaceutical aerosols is the inhaler, shown below. Inhalers contain metering valves on the aerosol opening. These valves are used to reproduce the necessary dosage expelled peruse. Inhalers are commonly used as portable spray treatments for asthma, COPD, and bronchitis.
Spray Bottles
Spray bottles, as shown below, consist of a plastic container capped with a fixture that acts as a pump. The fixture has a tube that extends to the bottom of the bottle. When the fixture is pressed, the concentrate is siphoned up the tube and passes through a nozzle at the top of the fixture. As the concentrate passes through the nozzle, it atomizes into smaller particles dispersed in the air creating a spray. Spray bottles are commonly used in nasal decongestant sprays.
Nebulizers
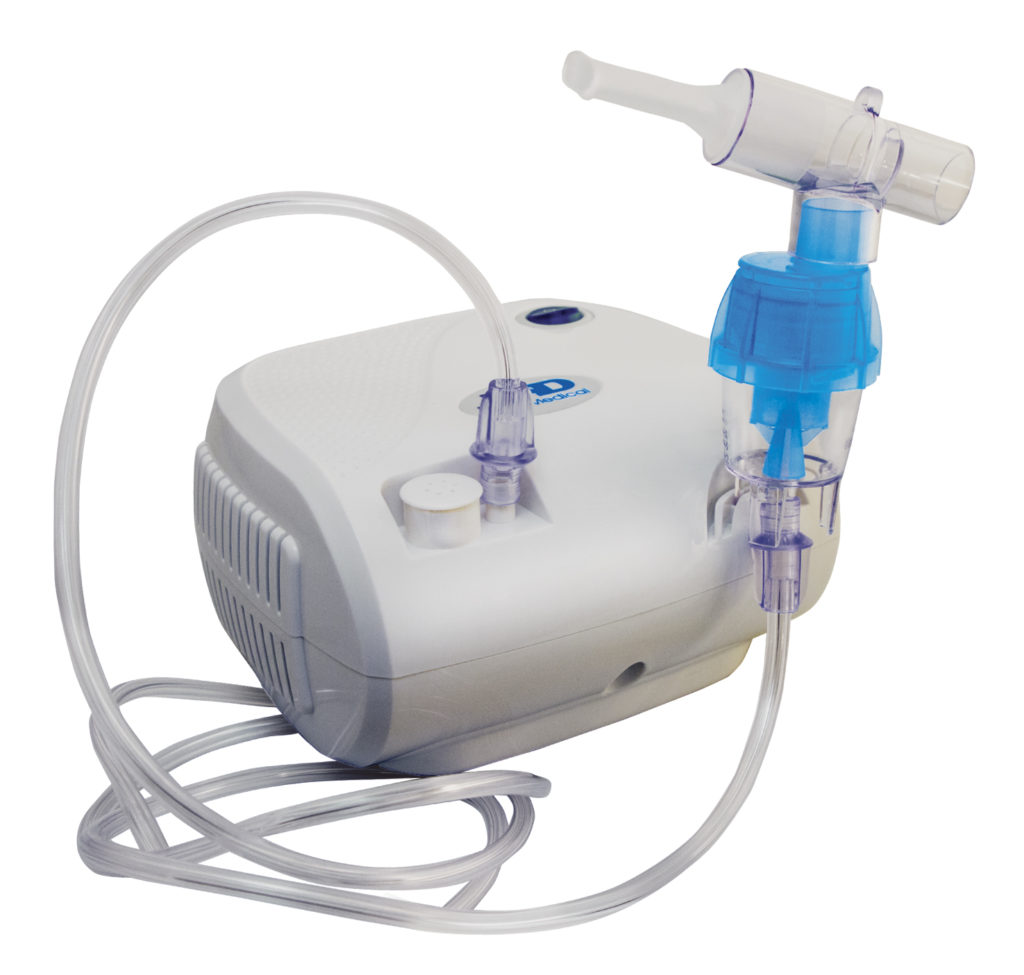
Nebulizers, shown below, differ from other sprays in that they are stationary, box-shaped devices, and the concentrate is often filled into the spray device from storage devices directly before use. There are two types of nebulizers, ultrasonic and air-jet. Ultrasonic nebulizers create a mist by vibrating the internal concentrate at a high frequency. Air-jet nebulizers feed pressurized air into the nebulizer, which mixes with the concentrate to create a mist. In both cases, the spray then transfers through a tube extending from the device where the patient can inhale the spray medicine. Nebulizers are typically used to deliver medicine to the respiratory system for hospitalized patients or for at-home use. They are commonly used for treating cystic fibrosis, asthma, and other long-term respiratory diseases.
Dry Powder Inhalers
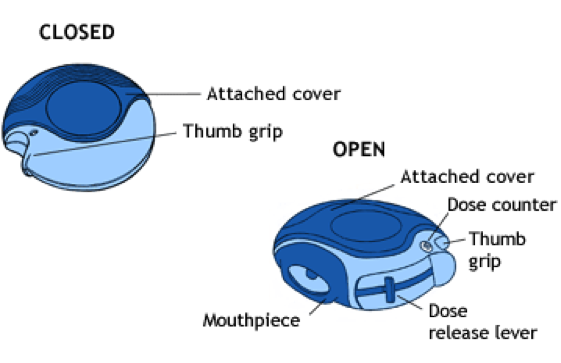
Dry powder inhalers (DPIs), shown below, are a type of spray device that delivers medicine to the respiratory system in the form of a dry powder. DPIs are disk-like devices that contain an internal, coiled strip with pockets containing one dose of powdered medicine. The coil rotates through the device after each usage. An external lever is pushed, causing one pocket of powder to line up with the mouthpiece. To administer the drug, the user inhales the powder through the mouthpiece. DPIs are commonly used to deliver powdered medicine for asthma, bronchitis, and other respiratory illnesses. DPIs have also been developed for inhalable insulin to treat diabetes.
After filling, sprays are ready for testing against product specifications. The Quality Control module describes equipment relevant to this testing process.
Acknowledgments
- A&D Medical, San Jose, CA
- Asthma Society of Canada, Toronto, Canada
- Berg Chilling Systems, Inc., Toronto, Canada
- GEA Process Engineering Inc., Columbia, MD
- JRPacking , Wuhan, China
References
- Bennett, Bill, and Cole, Graham. Pharmaceutical Production: An Engineering Guide. United Kingdom: The Institution of Chemical Engineers, 2002. Print.
- Ghosh, Tapash K., and Bhaskara R. Jasti. Theory and Practice of Contemporary Pharmaceutics. CRC, 2004. Print.
- Hickey, Anthony J., ed. Pharmaceutical Inhalation Aerosol Technology. 2nd ed. CRC, 2003. Print.
- Lipp, Charles W. “Sprays.” Kirk-Othmer Encyclopedia of Chemical Technology (2006): Web.
- Niazi, Sarfaraz K. Handbook of Pharmaceutical Manufacturing Formulations. Vol. 3. CRC LLC, 2004. Print.
- Sciarra, John J., and Christopher J. Sciarra. “Aerosol Technology.” Kirk-Othmer Encyclopedia of Chemical Technology (2012): Web.
Developers
- Nathan Hoffman