Distilled spirits are a type of beverage with a significantly higher alcoholic content than wine or beer. Also known as distilled liquor or “hard liquor,” this class of alcoholic drink encompasses brandy, rum, tequila, vodka, whiskey, and gin, among others. Distilled spirits are produced through yeast-driven fermentation of plants, fruit juice, or other carbohydrate-rich grains, followed by boiling or “distilling” (hence the name). All forms of alcohol undergo a fermentation phase, but at some point (generally between about 14-18%), the alcohol concentration becomes toxic to the yeast and thus further fermentation is halted. The distillation step is unique to spirits and is used primarily to increase the alcohol percentage in the final product to 30 percent and higher. Lastly, the mixture is given additives, filtered, sterilized, and packaged.
The defining phenomenon that enables the production of distilled spirits is the gap between the boiling points of water (100 degrees Celsius) and ethanol (78.5 degrees Celsius). Because of this gap, the mixture generated from fermentation (which is high in concentration of both water and ethanol) can be raised to a temperature intermediate between 78.5 and 100 degrees Celsius, which creates a two-phase separation of the mixture in which the liquid phase is enriched in water and the vapor phase is enriched in ethanol. The vapor phase (with a higher alcohol percentage than the initial mixture) is then condensed back into a liquid and then matured and/or mixed with additives to produce the desired flavor and consistency.
This article will give a brief overview of the process and equipment/machinery involved in preparation and fermentation, followed by a more comprehensive description of the distillation and additive/maturation steps of production of each distilled spirit.
Raw Materials
All distilled spirits are made from carbohydrate-rich ingredients, as carbohydrates are the substance on which yeast feeds and produces ethanol and carbon dioxide. The use of certain ingredients will, naturally, determine the type and flavor of liquor that is ultimately produced following distillation. Brandy is generally made from grapes, but can also be made from cherries, plums, apples, pears, and apricots. Rum is derived from sugarcane, either from molasses, honey, or juice extracted directly from sugarcane. Tequila is produced from the namesake agave tequilana plant. Vodka is generally synthesized from cereal grains, potatoes, or sugar beet molasses, but technically any vegetation with a high sugar or starch content can be used. Whiskey is produced from barley, wheat, corn, or rye grains. Gin is made from neutral grains and flavored with juniper berries (which in actuality are seed cones, not berries) and other botanicals.
Preparation
In order to maximize the efficiency of the fermentation step, the raw materials must be broken down and exposed as much as possible so that the yeast can access the carbohydrates present. The type of raw material determines the type of breakdown that it undergoes.
Pressing
For fruits used to make brandy, a softer process is used. Generally, the grapes or substitute fruit is pressed into a mash of juice and fruit pulp and skin. The three most common industrial processes are pressing through a filter or perforated surface, crushing with the use of rollers, and high-speed rotation (utilizing centrifugal force). The most commonly employed are the first two, whereas rotation is generally not as quick and cost-effective.
Milling
Milling is employed for any portion of production that requires the breakdown of tough vegetables or grains.
The first step in rum production is crushing or “milling” sugarcane to extract sugarcane juice. The pulpy waste is discarded or burned, and the sugarcane juice is either collected or concentrated into syrup or molasses. The product (usually molasses when producing rum) is now ready for fermentation.
In tequila production, the piña (or “core”) of the agave tequilana plant is removed after harvesting. Piñas are then gradually heated in large ovens in order to convert the complex polymeric fructose molecules (“fructans”) present into smaller, monomer fructose molecules. The baked piñas are then milled in some form of a mechanical crusher, such as a roller mill or hammer mill. This extracts the juice from the agave pulp, which is then filtered and collected.
Sugar beets or soft substitute fruits or plants used to make vodka can be milled and filtered to yield juice, which is then concentrated into molasses or syrup and can be immediately fermented. Tougher plants such as grains and potatoes are milled into a pulp-like substance to expose the starch polymers but must also be mashed in order to convert carbohydrates into simple, usable sugars.
For whiskey made from grains other than barley or wheat, milling is required to convert the grains into a meal. This meal is then cooked in water in order to break down cellulose and release starch molecules into the mixture. Barley and wheat undergo malting prior to mashing.
The base ingredient of gin is a neutral (flavorless) spirit, generally produced from grains that have been milled in a manner nearly or entirely identical to that used to make vodka. The spirit later becomes gin when it is redistilled with botanicals of choice (berries, seeds, spices, fruits, herbs, etc.).
Malting
Malting is the process by which certain grains (most commonly barley and wheat) are converted into a simple carbohydrate-and-enzyme-rich substance called “malt.” The grains are first soaked in water and then allowed to germinate in the open air on a “malting floor.” The grains are turned periodically in order to prevent temperature gradients, either manually or in large rotating drums.
During this germination, the amylase and diastase enzymes present in the grains are activated and convert the long starch molecules into simple sugars. After several days to a few weeks, the grains are then gently dried either with hot air or in a kiln to stop germination. It is important to halt germination so that the sugars in the grain are not entirely consumed. Now called “malt,” the germinated grains are ground down in a mill and then added to a batch of warm water for mashing, often with other enzyme-deficient grains to promote starch breakdown in them as well.
Mashing
Mashing is a step employed for some ingredients following milling when further breakdown is required, such as the raw ingredients used to make vodka and grains used to make whiskey. Mashing generally occurs in a mash tun, which is an industrial metal container that possesses rotors or blades for mixing and coils or a steam system for heating.
Grains or potatoes are first crushed and then heated with liquid water and/or steam, which causes the starchy sugars to form a gelatin. Next, the “mash” is then cooled to about 60 degrees Celsius, which is a suitable temperature for enzymes to break down the gelatinous starch into simple sugars. Barley and wheat generally contain a sufficient amount of the necessary enzymes (amylase and diastase), but potatoes and other grains generally need to be supplemented by another source.
Often this other source is malted barley and/or wheat, which possess sufficient amounts of the required enzymes. A concentrated powder or insoluble mixture (slurry) of amylase and diastase is added to the mash as a substitute in industrial or large-scale production. The malt or malt substitute is traditionally added to the mash tun along with warm water, and the reactions occur under controlled temperatures. The enzymes perform the final step in ingredient preparation, making the sugars in the mash ready for fermentation.
After the simple sugars from the grain mixture are given enough time to form and dissolve in the water, the liquid portion of the mash is filtered, and the solids are discarded or recycled as farm feed. The same batch may be filled with warm water and filtered several times in order to collect the maximum amount of sugar from the mash. The sugary liquid is collected and sent to the fermentation equipment.
Whiskey often uses only barley or wheat, so mixture with other grains and further enzymatic action is not required. Following extraction of the sugars in the warm water, the filtered liquid is called “wort” when pertaining to whiskey.
Fermentation
Following the preparation phase, the ingredients for each type of spirit have been reduced to a mixture rich in simple sugars. The specific process and yeast used for fermentation differ depending on the ingredients, the desired alcohol content, the flavors, and the scale of production.
The sugary fluid collected from pressing, milling, mashing, and or malting is placed in a large vat, wooden barrel, or stainless-steel tank. The container is either temperature-regulated itself or placed in a location with a steady climate. A temperature of 20 to 25 degrees Celsius is average, however, this can fluctuate depending on the type of fruit, flavor desired, etc.
It is important to maintain a constant temperature during fermentation so that the rate and reaction occur properly without generating unwanted characteristics.
For smaller, higher-quality batches, an “ambient yeast” is used, which is a yeast that naturally grows in the environment in which the spirit is being produced. For large-scale or industrial production, a “cultured yeast” is introduced into the sugary mash, which is a specifically isolated yeast suited for the production of the spirit in question.
The sugary mixture is left in the chosen container with yeast anywhere from a couple of hours to 14 days in “primary fermentation.” Sometimes another period, “secondary fermentation,” of 5 to 10 days follows the initial fermentation. Once complete, the resulting mixture is filtered and now ready for distillation.
Spirit-Specific Additional Processes
Often the yeast is released into a separate auxiliary tank filled with sugars in order to activate the yeast before fermentation. In this “propagation” or “starter” tank, the yeast reproduce rapidly, and subsequently, the yeast sample is much faster and more efficient at breaking down tougher sugar ingredients such as molasses.
When making whiskey, two types of fermentation may be performed: the sweet mash process or the sour mash process. Adding brand new yeast cells to the wort results in the sweet mash process. The fermentation must be rigidly controlled, occur quickly at a temperature of about 27 degrees Celsius, and foreign contamination prevented. The resulting “distiller’s beer” (in the U.S.) or “wash” (Scotland) is sweet to the taste, hence the name. The more commonly used sour mash process occurs when yeast is reused from a previous batch. The process is easier to perform since it can occur at room temperature and the acidity of the recycled yeast mixture is ideal for yeast growth and unfavorable to bacterial growth. The acidity gives the resulting distiller’s beer/wash the namesake sour taste.
Distillation
The fermented liquid (often called “wash”) then undergoes the final step of the process, distillation, which greatly increases the alcohol content in the mixture. Any distillation mechanism is made up of a still/retort (to hold the liquid during heating), a condenser (to lower the temperature of the vapors), a container (holding flavoring agents), and a receiving container (to collect the cooled liquid condensed from the distilled vapors). Distillation is generally carried out at about 175F (~79.5C) for all spirits but can be as high as 200F (~93C) in some cases.
There are two main types of distillation: batch and continuous. Batch distillation generally occurs in a pot still (a type of batch reactor) and is performed by loading and distilling the liquid in batches, hence the name. Continuous distillation involves the use of a distillation column (also called a fractionating column). The wash is fed in continuously and the distilled product (called the “distillate”) is removed as it is formed. Often the distillation column used is a rectifying column, in which the ethanol-rich vapor leaving the column is condensed and some of the distillate is fed back into the column. This is done to increase the concentration of ethanol in the exiting vapor.
Batch distillation is generally used for small quantities and higher quality spirits, especially whiskey and cognac. Continuous distillation is preferred when producing spirits on a large scale due to the ability to produce large quantities of product relatively quickly. The tradeoff is that it is more difficult to control the temperature and specific composition in continuous distillation. Thus, the quality of the spirits tends to be lower, and the taste is less uniform compared to spirits from a pot still.
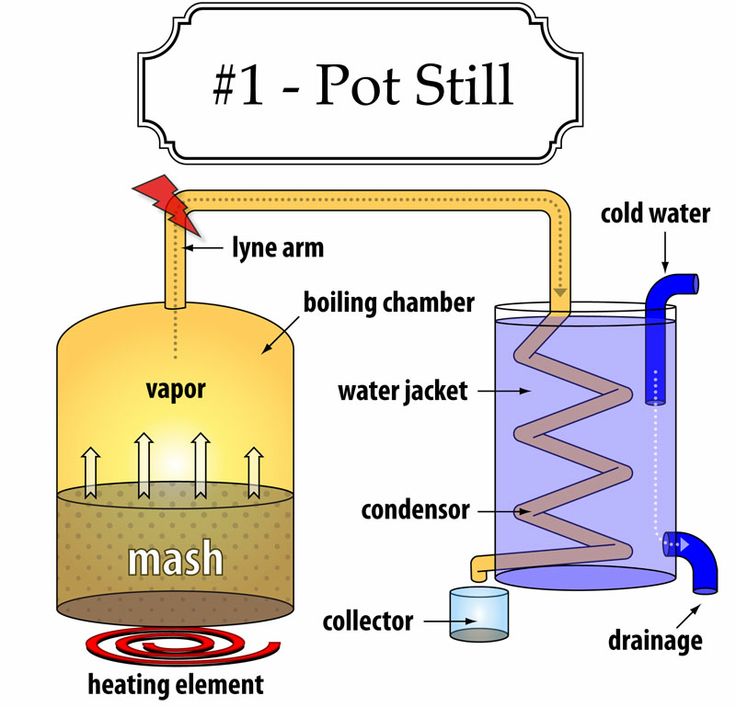
Pot Still
In a traditional pot still (or “alembic still”), the wash is loaded into the “pot” (also called the “boiling chamber” or “kettle”) via openings or pipes in the side of the container. The pot is typically made of copper and is heated either with fire at the base of the container or from electric coils around the outside of the container. At the top of the pot is the swan neck, which is a tapering pipe that extends vertically for several feet before abruptly turning downward. Often the pot still has a bulbous top, which serves to help condense undesirable substances before they leave the pot with the vapor. As the mixture boils, ethanol-rich vapors rise through the kettle and swan neck, where they are directed to a condenser. The condenser is generally a coil (or “worm”) of copper tubes in a countercurrent heat exchanger or water jacket. The distillate (now a cooled liquid) is collected.
Spirit-Specific Processes
The specific operation and processes employed in the distillation step vary based on the type of spirit being distilled. For most spirits, the first portion and last portion of the condensed vapor is removed and sent back to the distillation process, and only the middle portion (or “heart”) is retained. This heart can be the majority of the distillate or as little as only a third of the distillate for higher quality spirits.
Fine brandy production typically employs two rounds of distillation in a pot still. After the first distillation round, the distillate (called “low wine”) contains 20-30% alcohol. The middle third (or “heart”) of this is sent to the pot still again, producing a distillate of around 70-72% alcohol concentration. The middle third of this subsequent distillate is then sent to the next phase. Lower-quality brandies that are made in commercially large amounts are produced in a continuous distillation column. The distillate can have an alcohol concentration as high as ~97%.
Rum production also generally involves two rounds of pot still distillation, after which the product is usually between 70-95% alcohol. This “white” rum is then either bottled immediately or blended/aged in the following step. Rum from continuous distillation is also “white” and is generally lower in alcohol content.
Tequila is made in two (sometimes three) distillation rounds in a pot still. The first distillation is at a temperature of around 195F (95C) and takes about two hours and generates a roughly 20-25% alcohol concentration product, called “ordinario.” The second round of distillation is at a temperature of around 195F (95C) and requires three to four hours and yields “silver” or “blanco” tequila at an alcohol concentration of about 55%. Tequila is seldom made in a distillation column, but it is possible, albeit the quality is usually lower.
Vodka distilled in a pot still required at least two rounds, as the first produces a liquid with about 20-25% alcohol. Subsequent distillations can increase the alcohol content to around 70%. Fractional distillation in a column can produce vodka up to 95.6% alcohol, which is then usually blended with water.
In whiskey production, the wash from the fermentation step is distilled 2-3 times in a pot still. The liquid first enters the “wash still.” The produced vapors are condensed and this liquid (called “low wines” and having an alcohol content of about 20%) is then fed to the “spirit still.” The heart is at about 65-70% alcohol concentration and is sent to the next stage for aging. Whiskey is also mass-produced in distillation columns, following a nearly identical procedure as the other spirits.
Gin distillation can occur in two main ways: “steep and boil” or “vapor infusion.” In steep and boil, juniper and other desired botanicals are placed in a neutral spirit (generally around 50% alcohol) for up to two days. The liquid is then distilled in a pot still and the vapor is collected. In vapor infusion, the botanicals and juniper berries are suspended above the boiling neutral spirit such that the vapor comes into contact with them. Both of these processes can be carried out in a pot still or a distillation column. Sometimes a producer will utilize a hybrid of these two methods. Another option is to skip the second distillation, instead steeping the neutral spirit in botanicals for days or even weeks and then filtering out the solids and packaging the resulting liquid.
Maturation
Maturation is the final step in the production of distilled spirits prior to bottling and/or packaging. Maturation may involve aging and almost always involves blending.
Aging
Aging is the process by which harsh or undesired flavors are removed and desired flavors are added to spirits by allowing them to sit for a period of time in wooden barrels or casks (most often made of oak). Wood allows for evaporation while also dissolving somewhat into the spirit itself, giving the spirit a particular flavor. Aging is also the step wherein spirits attain their “dark” color, as the liquor is colorless and relatively flavorless when it first enters the barrel. Other types of wood can be used as well and the barrel may also be charred, both of which will alter the flavor and color that the spirit is imbued with during the aging process. The type of barrel selected also determines what flavors are removed from the spirit by the wood. The longer a spirit is aged, the higher the quality and flavor, and likewise the higher the commercial price.
The environment also plays a role in the aging process. The flavor of the spirit will vary as a result of the humidity, temperature, and salinity of the location in which it is aged. In addition, spirits generally lose 1-3% of their alcohol content for each year they are aged in cooler and wetter climates and can lose up to 6-7% per year in warmer and drier climates due to evaporation.
Aging is most commonly used (and often required) for whiskey and brandy, whereas vodka and gin are seldom aged. Rum and tequila are sometimes aged, but it is not required. There are several laws and regulations tied to spirits that determine how long they must be aged. All Scotch whiskey must be aged for a minimum of three years. In the U.S., whiskey can only be labeled as “straight bourbon” or “rye whiskey” if it has been aged in new charred white oak barrels. In other countries, barrels can be reused (and often are), sometimes even to make multiple types of spirits. Brandy is typically aged in barrels or casks for 2-5 years, but finer brandies can be aged for as many as 50 years. Rum and tequila do not have many minimums/requirements for their aging process, and often they are aged in white oak barrels that previously aged whiskey or brandy.
Blending
Blending is the action by which two or more components are combined so as to generate the final alcoholic product. Straight whiskeys and single malt Scotch are not blended. All other whiskeys are generally made up of several different whiskey batches, a plain neutral grain spirit, sherry and/or port wine (to help blend flavors), and caramel (to help with the amber coloring). Brandies, rums, and Joven tequilas are also made up of many different batches, along with distilled water (for dilution to the proper alcohol content), sugar (for flavor), and caramel (for color). Commercial vodka is diluted with distilled water as well, and often sugar, honey, glycerol, citric acid, and fruit flavorings are added to give the vodka flavor and the feel of “smoothness.”
Packaging
Because the components can easily evaporate, be extracted by, and react with containers and the surrounding environment, all distilled spirits are packaged in clean, sterile glass bottles following blending.
Acknowledgments
References
- Aldi, R., & Sequin, R. (2011, October 19). Distilled Spirits Industry. Retrieved from http://iloencyclopaedia.org/component/k2/109-65-beverage-industry/distilled-spirits-industry
- Aylott, R. (2003). GIN | The Product and its Manufacture. Encyclopedia of Food Sciences and Nutrition, 2889–2893. doi: 10.1016/b0-12-227055-x/00555-1
- Aylott, R. (2003). Whisky analysis. Whisky, 275–306. doi: 10.1016/b978-012669202-0.50026-9
- Buglass, A., & Caven-Quantrill, D. (2012). Applications of natural ingredients in alcoholic drinks. Natural Food Additives, Ingredients and Flavourings, 358–416. doi: 10.1533/9780857095725.2.358
- (n.d.). Distilled Spirits. EPA AP-42: Compilation of Air Emissions Factors, 9.12.3–1-9.12.3–6. Retrieved from https://www3.epa.gov/ttn/chief/ap42/ch09/final/c9s12-3.pdf
- Faria, J. (2012). Sugar cane spirits: cachaça and rum production and sensory properties. Alcoholic Beverages, 348–358. doi: 10.1533/9780857095176.3.348
- Katz, Sandor Ellix. “The Art of Fermentation.” Google Books. Chelsea Green Publishing, 2012.
- Palmer, G. (2016). Beverages, Distilled. Reference Module in Food Science. doi: 10.1016/b978-0-08-100596-5.00233-x
- Shipman, F. M., & Thomas, A. T. (n.d.). Distilled spirit. Retrieved from https://www.britannica.com/topic/distilled-spirit
- Szymczycha-Madeja, A., Welna, M., Jamroz, P., Lesniewicz , A., & Pohl, P. (2015). Advances in assessing the elemental composition of distilled spirits using atomic spectrometry. TrAC Trends in Analytical Chemistry, 64, 127–135. doi: 10.1016/j.trac.2014.09.004
Developers
- James Rivard