All pharmaceutical products must undergo quality control (QC) to ensure that the delivered product is up to the required specifications. This is very important for the pharmaceutical industry as minute deviations from a product specification, such as the quantity of API in the product, can have severe unwanted side effects for the end-user. The image below shows a spectrometer commonly used in the quality control of pharmaceutical products.
The following sections describe the regulations and equipment used for pharmaceutical quality control.
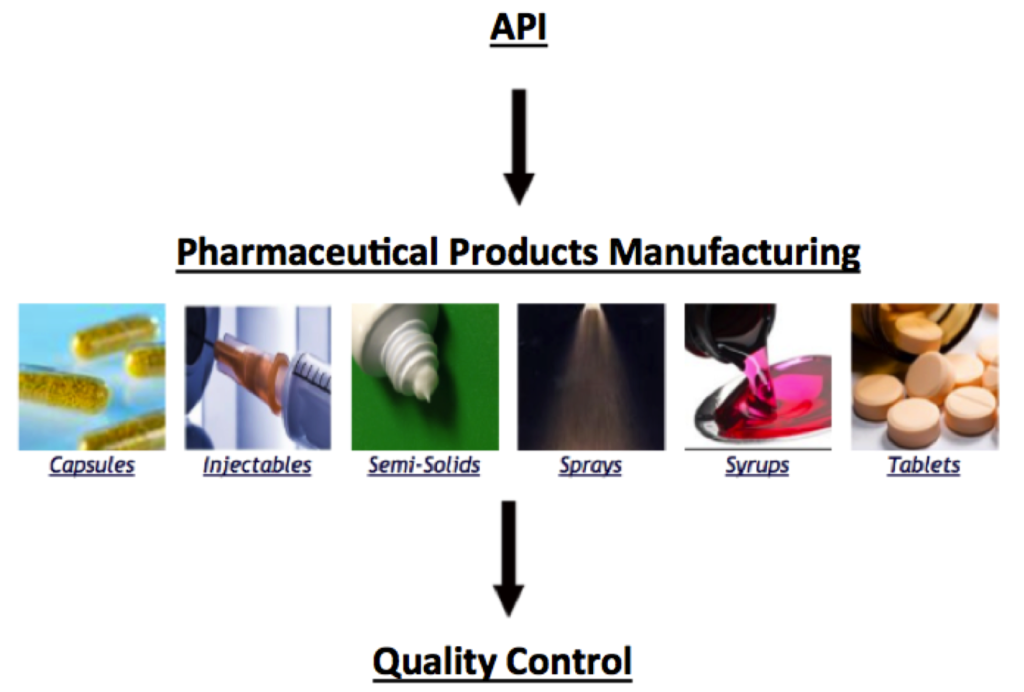
Pharmaceuticals Process Map
Importance of Pharmaceutical Quality
Unfortunately, quality control has not always been held to a high standard in the U.S. pharmaceutical industry. Until 1906, when the Pure Food and Drug Act was passed by Congress, many pharmaceutical companies would brand drugs that either contained inactive or dangerous ingredients rather than API. This bill enabled the government to prevent the manufacture and commerce of misbranded drugs.
In 1941, a manufacturer of sulfathiazole, a topical and oral antimicrobial drug, distributed tablets tainted with phenobarbital, an anti-seizure sedative. This contamination resulted in about 300 combined deaths and injuries. This quality disaster prompted the Food and Drug Administration (FDA) to increase quality requirements, creating what is known today as Current Good Manufacturing Practices (CGMPs).
Since the creation of CGMPs in 1978, pharmaceutical quality in the manufacturing setting has greatly improved. However, many pharmaceuticals can still cause severe negative side effects or even death to patients if the drug is administered in incorrect dosages or is contaminated. As a result, quality control remains one of the major focuses of pharmaceutical manufacturing.
Quality Control Regulations
The current regulations for pharmaceutical manufacturing are referred to as the Current Good Manufacturing Practices (CGMPs). These regulations are enforced by the FDA.
CGMPs require that pharmaceutical manufacturers assure the correct chemical identity, overall quality, and purity of pharmaceutical products. They include requirements for pharmaceutical companies to establish quality management systems, obtain suitable raw materials, establish detailed operating procedures, and maintain reliable testing laboratories to meet these assurances. Manufacturers must also have a system for detecting and investigating quality deviations. These requirements, if met properly, are meant to prevent contamination or errors in pharmaceutical product quality. Pharmaceutical operations must meet the current CGMPs to comply with FDA regulations. So, companies must often upgrade in-process or quality testing equipment to maintain compliance.
Quality Control Equipment
Equipment relevant to pharmaceutical quality control is used to either monitor the manufacturing process, test pharmaceutical products against the product specifications or validate the sanitation of the process.
Various process parameters equipment is used to monitor pharmaceuticals during processing. This equipment is used to record process parameters as part of the CGMP requirement to detect and investigate quality deviations. For example, humidity measurement is used to monitor capsule and tablet production as high humidity will degrade the finished products. Turbidimeters are also commonly used in liquid pharmaceutical processes to detect any microbial presence in process liquids to prevent contamination. Other process parameters may be monitored closely if they are known to be imperative to product quality or safety. As a result, the exact parameters monitored vary for each pharmaceutical process.
After production is complete, the product is tested against the specifications to assure chemical identity, quality and purity are adequate for human use. Pharmaceuticals are tested using process parameters equipment in a reliable laboratory. The chemical identity and purity of the finished product are confirmed by testing on spectrometers and/or chromatography columns. This is commonly done for products that were sterilized by heating to confirm the API did not degrade during heating. Additionally, liquid pharmaceutical products can be tested for other parameters critical to quality and safe consumption, such as pH and viscosity. pH measurement devices and viscometers are used to complete these tests. Pharmaceutical products can also be tested to specification for many other parameters depending on their importance to the product quality and safety.
In addition to in-process equipment, pharmaceutical manufacturers regularly conduct extra testing to validate that the process is free of contamination from unwanted chemicals or microbial life. Spectrometers are commonly used to test for contamination on lab surfaces. Additionally, chromatography columns and pH measurement devices are often used to test for contamination or microbial growth on surfaces in production areas. Regular process validation is essential to pharmaceutical quality control as sources of contamination can be found and addressed before product contamination affects the end-user.
Acknowledgements
- Aurora Biomed Inc., Vancouver, B.C.
References
- “Manufacturing – Facts About the Current Good Manufacturing Practices (CGMPs).” U.S. Food and Drug Administration. 6 Jan. 2015. Web.
- Rzeszotarski, W. J. “Pharmaceuticals.” Kirk-Othmer Encyclopedia of Chemical Technology. 2005. Web.
- Sarker, Dipak K. Quality Systems and Control for Pharmaceuticals. Wiley, 2008. Print.
- Wang, H.Y, personal communications, 2016.
Developers
- Nathan Hoffman