Adsorption involves the separation of a substance from one phase, accompanied by the accumulation of that substance at the surface of another phase.

(Copyright Water Technologies Business Unit of
Siemens Industry, Inc., Warrendale, PA)
Adsorption Theory
This animation exemplifies the basic process of adsorption. Material from the liquid phase is concentrated on the surface of the solid.
The adsorbing phase, which is a solid in the animation above, is called the adsorbent, and the material being adsorbed at the surface of that phase is the adsorbate. Note that adsorption is different from absorption, a process that involves the interpenetration of one material into the bulk of another.
Two types of adsorption are possible. The first, physisorption, is a physical process that occurs below 200°C. As the animation below demonstrates, the material is adsorbed due to molecular interactions between the adsorbent and the adsorbate. The typical heat of physisorption is 5 – 10 kcal/mol.
The second type of adsorption, chemisorption, is a chemical process where the adsorbent adheres to the adsorbate through a chemical bond. As the animation below demonstrates, adsorption occurs due to the formation of chemical compounds. Chemisorption bonds can be weak, ranging from 15-40 kcal/mol, or strong, which can be greater than 50 kcal/mol.
Adsorption Systems
Column Contact
Column contact adsorbers use a bed of adsorbent to purify solutions. The packed bed columns shown below are used in a natural gas dehydration plant.

(Copyright Bertsch Holding GmbH, Austria)
General Information
Column contact adsorbers can operate as a fixed bed, or as a moving or pulsed bed.
Fixed bed operation, shown below, is the oldest form of column contact adsorption. A bed of adsorbent is held in place inside the column, and the solution to be treated flows over, through, and around it. The bed must be taken offline to replace or regenerate the spent adsorbent.
In a moving or pulsed bed adsorber, the untreated solution enters the adsorber from the bottom and flows up the column. At the same time, a fresh adsorbent enters the adsorber from the top of the column and exits out the bottom. Exhausted adsorbent is continually removed, while fresh adsorbent is continually added, allowing for more efficient operation.
Equipment Design
Under fixed bed operation, adsorption columns may be arranged in series or parallel and maybe run in either upflow or downflow modes.
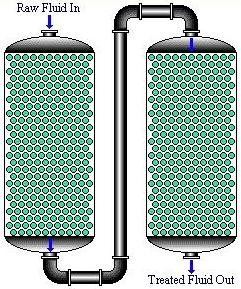
In series fixed-bed column contact mode, the effluent from the first bed passes to a second bed. If necessary, additional beds may be added in series. The lead bed is removed for reactivation when the adsorbent becomes saturated with an adsorbate. The next bed in sequence then becomes the first bed, and a fresh bed is added in the final position.
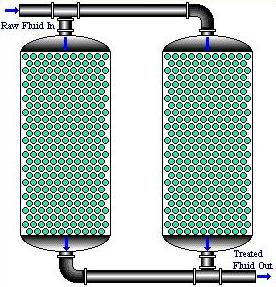
In parallel fixed bed operations, the effluent of all columns is blended prior to discharge. Adsorption beds in parallel are removed from operation in a staggered manner so that the system is comprised of beds in varying states of exhaustion.
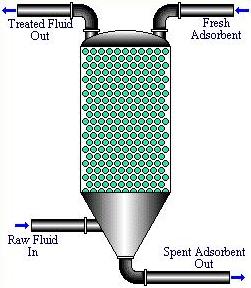
Pulsed bed operations are restricted to the upflow mode of operation. Additional equipment is required to recycle the adsorbent, which allows for a more efficient operation.
Smaller adsorbents have a larger surface area and allow for more contact between the packing and absorbate than larger adsorbents. Therefore, small adsorbents are desirable and enhance the adsorption rate. However, if adsorbent particles are too small they may restrict the proper flow of fluid through the column.
Usage Examples
Column contact adsorbers are used in various industries, such as sugar refining and petroleum processing. Parallel fixed bed operations are used in areas in which high accuracy is imperative, such as in the production of pharmaceuticals or the processing of wastewater. This system is also common for large flow rates. An adsorption column used to purify gas streams in laboratory settings is shown below.

Drierite Co., Xenia, OH)
Advantages
- Series fixed beds: lead bed exhausted more fully than one large single fixed bed.
- Parallel fixed beds: may ensure average concentration of total effluent flow meets contaminant concentration requirements in the discharge for uneven feed rates.
- Pulsed beds: total column shut down not required to replace or regenerate adsorbent.
Disadvantages
- Additional equipment required for pulsed beds.
- Fixed parallel operation is inefficient.
- Fixed beds require total or partial shutdown to replace adsorbent.
Slurry Contact
Slurry contact adsorbers use a powdered adsorbent slurry to adsorb desired materials. Shown below are slurry contact adsorbers used in the production of hydrochloric acid.

Pittsburgh, PA)
General Information
In slurry contact adsorption, the adsorbent powder is mixed with the solution that is to be treated. Agitation evenly distributes the adsorbent throughout the solution. The adsorbent is then removed from the purified solution by filtration.
Slurry contact adsorption can be carried out in a number of modes, such as single stage batch, multiple stage batch, multiple stage countercurrent, and continuous.
Equipment Design
In single-stage batch treatment, fresh adsorbent comes in contact with the fluid in a completely mixed vessel. After the required contact time, the adsorbent is separated from the fluid by filtration. The desired quality of the purified fluid is obtained, and the spent adsorbate is disposed of or regenerated.
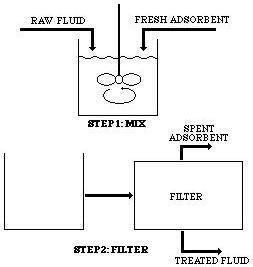
In multiple-stage batch treatment, the solution passes through several single batch stages. The effluent from one stage enters the second stage, where it is treated again. Each stage achieves part of the overall separation in this type of treatment, also known as split or divided treatment.
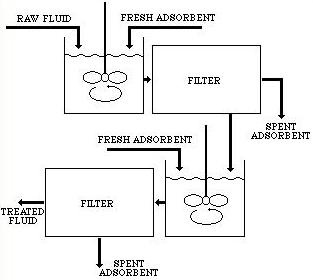
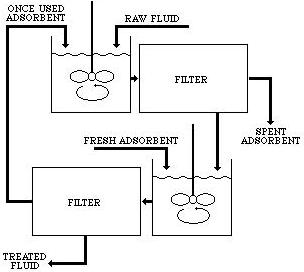
Multiple stage countercurrent adsorption separation is a two-step system. It involves contacting the untreated solution with a once-used adsorbent, which is discarded or regenerated after the second use.
The partially treated fluid is then contacted with a fresh adsorbent. After separation, this adsorbent becomes the once-used adsorbent to treat a new batch of untreated feed solutions.
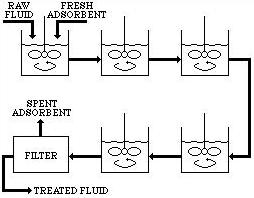
In continuous slurry contact adsorbers, a series of stirred tanks are used to approach plug flow. The adsorption takes place as the adsorbent and solution travel through the series of tanks. When the desired purification has been achieved, the adsorbent is filtered from the purified solution.
Usage Examples
Some common applications of slurry contact adsorption include water purification, pharmaceutical applications, and decoloring. An activated carbon VOC recovery system is pictured below.

Corporation, Pittsburgh, PA)
Advantages
- Countercurrent contact can improve cost-effectiveness.
- Shut down is not necessary to maintain fresh catalyst.
Disadvantages
- Multiple-stage processes are more expensive than single-stage counterparts.
- Additional filtration equipment is needed.
- Without regeneration, the use of powdered adsorbent could be expensive.
Pressure Swing
In pressure swing adsorption (PSA), adsorbents are used under varying pressures to separate gas mixtures. The process is known as vacuum swing adsorption (VSA) if these pressures fall below atmospheric.

General Information
In pressure swing adsorption, one or more components in a gas stream adsorb on a solid adsorbent. PSA capitalizes on the dependence of adsorption on pressure. More gas can be adsorbed at higher pressures. By adsorbing at one pressure and then “swinging” to a lower pressure for desorbing, a majority of an adsorbed gas can be removed from a high-pressure feed.
PSA can be accomplished using one-, two-, or multiple-bed designs. However, two-bed designs are the most common.

Newington, CT)
In vacuum swing adsorption the operating pressures fall below atmospheric, so vacuum pumps are used. The choice between VSA and PSA depends on the pressure of the feed. VSA is used for low-pressure feeds, while high pressure feeds usually require PSA. Oxygen is usually used in low-pressure applications and can be produced by VSA units.
Equipment Design
The animation below shows what happens in the adsorption beds. In the two-bed design, the beds alternate periods of high and low pressure. The feed gas, shown in orange, enters the bed when the pressure is high. Components from the stream are preferentially adsorbed; to demonstrate this, adsorbents change color from white to red in the animation. A small part of the exiting stream, shown in brown, is used to desorb the collected species from the low-pressure bed. The adsorbents change in color from red to white to demonstrate this. The low-pressure bed can now become the high-pressure bed, and the cycle can continue using the opposite beds.
This schematic shows a typical PSA unit. The main components are the two adsorber beds, the product tank, and the feed and vacuum blowers pumps or compressors that help regulate the pressure of and flowrate through the two beds. In VSA units, the pumps or compressors would be replaced with feed and vacuum blowers. The system is automated, with computer-controlled switching valves to direct flow. The residence time within the bed will partly determine the product purity.
Usage Examples

Pressure swing adsorption is a popular technique used to separate components of a gas stream cost-effectively. It is applied in many industries, such as chemical processing, petroleum, medical, and specialty gases. Equipment size can vary from small indoor equipment, as in the medical system shown on the left, to large outdoor equipment, such as the nitrogen inerting tanks on the right.


(Copyright On Site Gas Systems, Inc., Newington, CT)
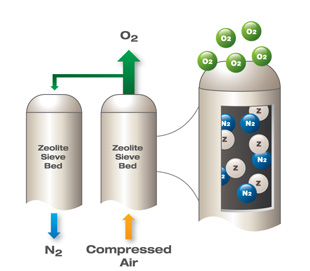
Pressure swing adsorption is also used for air separation. Special adsorbents known as zeolites have been developed to preferentially adsorb the nitrogen, water, and carbon dioxide in the air and allow the oxygen to be separated. Ambient air is pumped in and compressed before being sent to the adsorption beds. The waste gases are desorbed under a vacuum in VSA oxygen plants. The oxygen product can be used in many applications such as combustion or water treatment, as shown below.

(Copyright On Site Gas Systems, Inc., Newington, CT)
The pressure swing adsorption unit shown below uses multiple beds to separate the hydrogen and carbon monoxide in synthesis gas at a refinery and petrochemical plant.

(Copyright On Site Gas Systems, Inc., Newington, CT)
The system works in the same way as the others, with a high-pressure adsorption and low-pressure desorption process.
Advantages
- Inexpensive to operate compared to other gas separations.
- Can produce up to 99% pure streams.
- Selective adsorbents to separate mixtures.
Disadvantages
- Compression and expansion while changing pressures can be noisy.
- Leakage can be a problem during the opening and closing of valves.
Adsorption Media
Adsorbents
A wide variety of adsorbents are available to carry out specific separations.
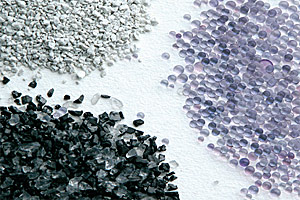
Delta, B.C., Canada)
General Information
Adsorbents are made from natural or synthetic materials and have an amorphous or microcrystalline structure. They are granular and generally extremely porous, with large internal surface areas. Examples of adsorbents include clays, chars, alumina, and silicates.



(Copyright Adsorbents and Desiccants Corporation of America, Los Angeles, CA)
Equipment Design
Both the chemical and physical properties of the adsorbent must be considered. Chemical properties that influence adsorbent design include the degree of ionization of the surface, functional groups present on the surface, and the degree to which these chemical properties vary with process parameters and by contact with the solution. Physical properties that influence design include surface area, surface structure, size, and pore distribution.
Pictured to the left are a molecular sieve and an activated carbon desiccant. The picture on the right shows a molecular sieve adsorbent.


When the adsorbent becomes saturated with adsorbate, it is either discarded or regenerated.
The adsorbent is typically discarded when chemisorption takes place since the adsorbent has undergone an irreversible chemical change. After physisorption, the adsorbent may be regenerated by heating it to high temperatures.
The picture below shows a desiccant made from silica gel.

Corporation of America, Los Angeles, CA)
There are many types of adsorbent materials, including activated carbons, synthetic polymeric materials, carbonaceous materials, nanoporous polymers, aluminophosphates, and alluminosilicates.

Havre de Grace, MD)
Activated carbons, shown above, are the oldest and most widely used adsorbent. They are natural, broad-based adsorbents with a wide range of applications. Activated carbon is highly porous, has high surface area adsorptive properties, and has a largely amorphous structure, making it a favorable adsorptive material choice. The pictures below show close-up views of the pores within activated carbon from coal, coconut shell, and wood respectively.

Coal

Coconut Shell
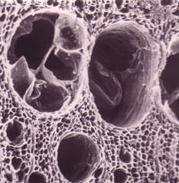
Wood
(Copyright Cameron Carbon, Inc., Havre de Grace, MD)
Carbonaceous adsorbents present a carbon matrix in a nonconventional manner. One of the more exotic and recently developed carbonaceous adsorbents is the carbon fullerene structure, also known as a Buckyball, shown below.

Released under the Gnu Free Documentation License)
Synthetic polymeric adsorbents, such as the one pictured below, are offshoots of synthetic ion exchange resin technology. Polymeric adsorbents have a fixed pore structure in a three-dimensional matrix.

Nanoporous polymers can accommodate various adsorbates because of their permanent porosity in the crystal structure. Nanoporous polymers are also good thermal insulators because their small pore size reduces collisions between gas molecules.
Aluminophosphate (ALPO) porous materials are a framework of aluminum, phosphorus, oxygen, and up to 17 other elements. Transition metals can be added to the framework to create different adsorption properties. Shown here is an electron microscope image of a silicoaluminophosphate (SAPO) material, one common derivative of ALPO.

University of Puerto Rico – Mayguez, Mayaguez, PR)
Aluminosilicates or zeolites are tetrahedral coordination of silicon, aluminum, and oxygen atoms. The arrangement of the atoms forms a framework of uniform pore channels, shown here. Aluminosilicates are negatively charged and cations are needed to compensate. Differently charged species can be used to produce specific adsorption interactions.
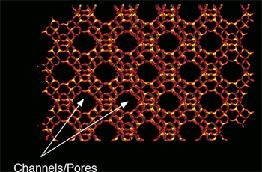
University of Puerto Rico – Mayguez, Mayaguez, PR)
Usage Examples
Adsorbents have a wide variety of uses. Some activated carbons are used in the military for defense against gas warfare. Activated carbon cartridges are placed in gas masks and adsorb harmful gases. The activated carbon used has 1,000,000 square meters of surface area per kilogram of adsorbent.
Activated carbon is also used in biogas purification and sediment remediation. Biogas produced at landfills, wastewater treatment facilities, or animal manure contain siloxanes and volatile organic compounds ( VOCs ) – that can be removed by activated carbon through physical adsorption. For in-situ remediation of contaminated sediments, activated carbon amendments are placed on contaminated sediments to remove polychlorinated aromatic hydrocarbons (PAHs) as well as polychlorinated biphenyls (PCBs).
Silica gel is often contained in small packages, as exemplified in the images below. It is used as a drying agent in various industries, such as electronics and food.

(Copyright Desiccare, Inc., Pomona, CA)
Other examples of adsorbents include Fuller’s Earth and Bauxite. Fuller’s Earth is used in petroleum refineries and is also used to purify vegetable and animal oils. Bauxite is an adsorbent used to dehydrate gas streams.
Advantages
- Activated carbon, silica gel, and aluminosilicates are inexpensive.
- Can be synthesized and functionalized for specific applications.
- Regeneration possible after physisorption.
- Cheaper than pumping or dredging for in-situ remediation.
Disadvantages
- Unwanted components may be adsorbed on the surface of adsorbents in place of the desired adsorbate.
- Energy-intensive regeneration after chemisorption is not cost-effective.
Acknowledgements
- Adsorbents and Desiccants Corporation of America, Los Angeles, CA
- Air Products and Chemicals, Inc., Allentown, PA
- Bertsch Holding GmbH , Austria
- Buyers Packaging Group Ltd., Delta, B.C., Canada; part of Crown Packaging
- Calgon Carbon Corporation, Pittsburgh, PA
- Cameron Carbon, Inc., Havre de Grace, MD
- Delta Adsorbents, Roselle, IL
- Desiccare, Inc. , Pomona, CA
- Dry Pak, Encino, CA
- Hernandez, Arturo J., Chemical Engineering Department at the University of Puerto Rico, Mayaguez, PR
- Linde AG Engineering Division, Pullach, Germany
- Michael Strock
- Novasep , Pompey, France
- On Site Gas Systems, Inc., Newington, CT
- W. A. Hammond Drierite Co., Xenia, OH
- Water Technologies Business Unit of Siemens Industry, Inc., Warrendale, PA
References
- Cleveland, T.G.; Garg, S., Journal of Environmental Engineering. March 1996 pp. 235-238.
- LaCava, A.I., Ramachandran, R., Shirley, A.I., “How To Specify Pressure-Swing Adsorption Units,” Chemical Engineering, June 1998, pp. 110-118.
- Koehlert, Ken. “Activated Carbon: Fundamentals and New Applications.” Chemical Engineering. July 2017: 33, 35. Print.
- Mantell, C. L. Adsorption. 2nd ed. New York: McGraw-Hill, Inc., 1951.
- Rousseau, Ronald W. Handbook of Separation Process Technology. New York: John Wiley & Sons., 1987. 659-688.
- Schweitzer, Philip A. Handbook of Separation Techniques for Chemical Engineers. 2nd ed. New York: McGraw-Hill Inc., 1988. 1-515 – 1-519, 3-3 – 3-47.
- Slejko, F.L. Adsorption Technology: A Step by Step Approach to Process Evaluation and Application. New York: Marcel Dekker Inc., 1985.
- Yang, R.T. Adsorbents: Fundamentals and Applications. Wiley: New York, 2003.
Developers
- Sam Catalano
- Jason Lawrence
- Chris Seadeek
- Joseph Palazzolo
- Steve Wesorick
- Steve Cotton
- Emma TerBeek