Many organic compounds are combusted into carbon dioxide and water in oxidizers. Combustion is an exothermic oxidation reaction that destroys VOCs before emission.

(Copyright A.H. Lundberg Systems Limited, Vancouver, B.C.)
General Information
Oxidizers, such as the one shown here, combust feed streams to produce environmentally safe products. The oxidizers rely on high temperatures and burners for complete combustion and result in 99% oxidation efficiency. The heat released from the reaction is recaptured by the oxidizers, making them more efficient. There are three types of oxidizers: thermal, catalytic, and regenerative.

(Copyright A.H. Lundberg Systems Limited, Vancouver, B.C.)
Equipment Design
Thermal Oxidizers
In thermal oxidizers, the gas stream enters the oxidizer, as shown by the blue arrows in the schematic, and flows into a pretreatment section, where it is heated to the reaction temperature using heat exchangers. Once it enters the reaction section, the feed gas mixes with fuel in the burner, and combustion occurs. The heat released by the combustion is recaptured by the heat exchangers, and the clean gas is released into the atmosphere. Pictured on the right is an operating thermal oxidizer that handles a volumetric flow rate of 5,000 standard cubic feet per minute (SCFM).
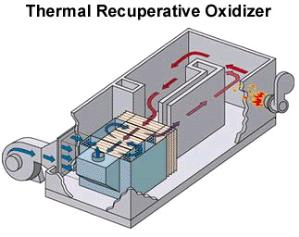

(Copyright Anguil Environmental, Milwaukee, WI)
Catalytic Oxidizers
In catalytic oxidizers, catalysts, such as platinum, are used to enhance the reaction, resulting in lower operating temperatures. This lowers the energy requirements for the process. The design of the oxidizer, as shown here, is similar to the thermal oxidizer. The only major difference is that catalyst beds are used in the reaction zone. The catalytic oxidizer pictured on the right operates at 4,000 SCFM.
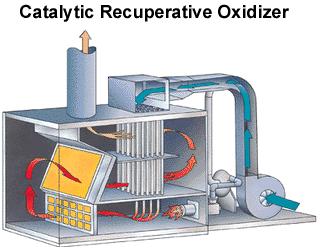

(Copyright Anguil Environmental, Milwaukee, WI)
Regenerative Oxidizers
Regenerative oxidizers have a different design than the other two types of oxidizers, as seen in this schematic. The feed is split and enters the oxidizer in two places. The feed flows up through beds of heat-recovery media, such as ceramic heat transfer blocks, and heats up to the reaction temperature. The warmed feed then enters the reaction zone and passes a burner, where combustion occurs. The product stream then exits the column. The heat generated by the exothermic combustion reaction is recovered by the media and used to preheat the incoming feed. The regenerative oxidizer pictured on the right operates at 5,000 SCFM and has stainless components.
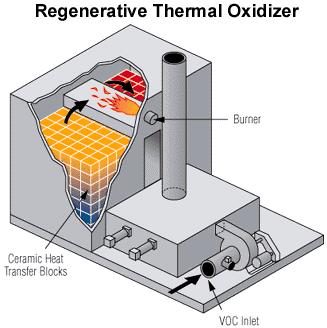

(Copyright Anguil Environmental, Milwaukee, WI)
Usage Examples
Oxidizers are used for air pollution control in a variety of industries, from the automotive industry to chemical plants. The picture here shows an oxidizer used for VOC and odor control at a textile plant.

(Copyright Glenro Inc., Paterson, NJ)
Advantages
- Much of the heat from the exothermic reaction can be recovered.
- Almost complete destruction of organic contaminants.
- Simple operation.
Disadvantages
- Potential for explosion.
- Combustion may produce products, such as SO2, that need further processing.
- High operating costs.
Acknowledgments
- A.H. Lundberg Systems Limited, Vancouver, B.C.
- Anguil Environmental, Milwaukee, WI
- Catalytic Products International, Lake Zurich, IL
- The CMM Group, LLC., De Pere, WI
- Glenro Inc., Paterson, NJ
- Saing-Gobain NorPro, Stow, OH
References
- Perry, Robert H. and Don W. Green. Perry’s Chemical Engineers’ Handbook. 7th ed. New York: McGraw-Hill, 1997: 25-5; 25-8; 25-22 – 25-57. Print.
Developers
- Chris Seadeek
- Kelsey Kaplan
