Gas and flame detectors determine the presence of a gas or fire, respectively. Gas monitors check or regulate the amount of gas present. The gas detectors pictured below detect carbon monoxide and hydrogen sulfide.
While detectors and monitors serve different purposes, they are configured similarly. The main elements of both detectors and monitors are a transmitter and a sensor. The distinguishing difference in both monitoring and detection systems is the type of sensor that the system uses. Catalytic, electrochemical, and infrared sensors and their corresponding transmitters are the three main types of sensors used in monitoring and detection systems.

Catalytic
Catalytic sensors are the most commonly used sensors in the offshore oil and gas industries.

General Information
Catalytic detectors are based on the chemical oxidation of a combustible gas on a heated bead coated with catalyst to produce heat. The picture below shows the heated catalytic beads.

Equipment Design
In a catalytic sensor both a reference bead and an active bead, pictured below, are held at a constant temperature. The chemical oxidation of a combustible gas on the active bead creates a temperature increase. This temperature increase changes the resistance of the wire, which is usually made of platinum. This change in resistance is related to the gas concentration.
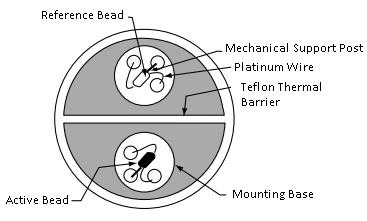
Usage Examples
Catalytic sensors are most commonly used to detect and monitor combustible hydrocarbon levels in the offshore oil and gas industries.

Advantages
- Low in cost.
- Easy to calibrate and service.
- Can be replaced easily.
- Small.
- Can be made mechanically rugged.
- Long life (usually 2-4 years).
- High reliability.
- Versatile in a wide range of gases.
Disadvantages
- Catalyst can be poisoned by certain compounds (e.g. silicon).
- Loss of sensitivity when exposed to high gas concentrations.
- Frequently miss small leaks.
- Requires constant supply of oxygen.
Infrared
Infrared sensors are used in hydrocarbon gas monitors and detectors. In addition, flame detectors commonly utilize an IR sensing mechanism.

General Information
Infrared sensors measure two wavelengths, a reference and a sample wavelength. The ratio of the sample wavelength energy to the reference wavelength energy indicates gas concentration. Pictured here is an IR detector for hydrocarbon gas detection applications.

Equipment Design
The schematic below shows the basic configuration of a single-path, dual wavelength IR detector. If the gas being monitored or detected is present between the mirror and the main detector the sample wavelength will be absorbed and the reference wavelength will not be absorbed. This difference in radiation indicates the gas concentration.
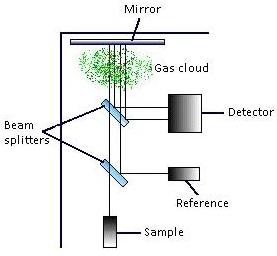
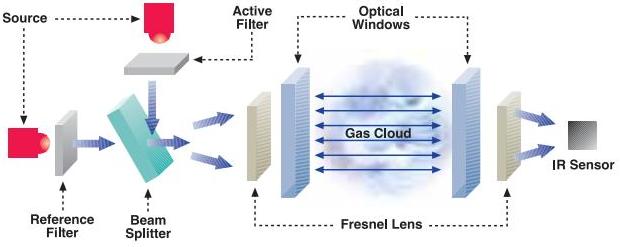
In a dual-beam detector, sources generate pulses of two optical beams, an active and reference beam, which are then guided to follow the same optical path though the cloud of gas. Both beams follow the same path, but the reference beam contains radiation at a wavelength not absorbed by the gas. The IR sensor measures and compares the intensity of the two wavelengths to determine the gas concentration.
Usage Examples
In addition to gas detection and monitoring, infrared sensors are also used in flame detectors because they can detect the infrared radiation emitted by flames. Specifically, they respond well to hydrocarbon fueled fires. Pictured here is an IR flame detector.

Advantages
- Can be made specific to a particular gas.
- Require less calibration than other sensors.
- Relatively maintenance free.
- Does not require oxygen.
- Freedom from poisoning.
- No loss of sensitivities.
- Quick response.
Disadvantages
- Dust and dirt can coat optics and impair response.
- Not well suited for multiple gas applications.
- Can be affected by humidity and water.
- High initial cost.
- Cannot monitor all gases.
Electrochemical
General Information/Equipment Design
Electrochemical sensors contain two active electrodes in a casing: the working electrode (anode) and the counter electrode (cathode). The top of the casing has a membrane which the gas sample permeates. Chemical changes take place at the electrodes and the charge is conducted through the sample phase or gel within the casing. The electrode reactions and the charge transport indicate whether or not a toxic gas is present as well as the relative amount present. Pictured on the left is a sulfur dioxide electrochemical sensor, which can attach to a transmitter, such as the one shown on the right.


Usage Examples
Electrochemical sensors are most commonly used to detect and monitor toxic gas and oxygen in refineries, such as the one pictured here, and in chemical plants.

Advantages
- Can be specific to a particular gas.
- Accurate.
- Do not get poisoned.
- Monitor at ppm levels.
Disadvantages
- Narrow temperature range.
- Short shelf life.
- Subject to interfering gases (e.g. hydrogen).
- Sensor lifetime will be shortened in very dry, hot areas.
Acknowledgements
- Analytical Technology, Collegeville, PA
- Delphian Corporation, Northvale, NJ
- General Monitors, Lake Forest, CA
- Honeywell Analytics Inc., Lincolnshire, IL
References
- Austin, Alan. “Comparing Catalytic vs. Infrared Gas Monitors.” Chemical Engineering 1 (2002): 75-78. Print.
- Janata, Jiri. Principles of Chemical Sensors . New York: Plenum, 1989. 81-240. Print.
- Lloyd, Adrian. “Protect Your Plant with Fire and Gas Detectors.” Chemical Engineering Progress 92 (Oct 1996): 74-78. Print.
- Nyboe, Hans Olav and Tor Onshus. “Gas Detectors Move to the Third Dimension.” Intech 43 (April 1996): 36-39. Print.
- Wang, Joseph. “Electrochemical Transduction.” Handbook of Chemical and Biological Sensors. Ed. R. F. Taylor and J. S. Schultz. Bristol: Institute of Physics Pub., 1996. 123-137. Print.
Developers
- Melissa Schlosser
- Matthew Robertson
- Kelsey Kaplan

