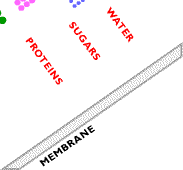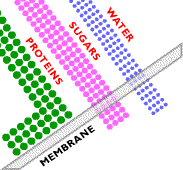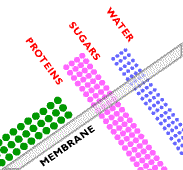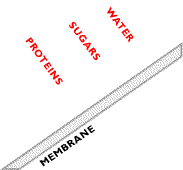
A membrane is a thin barrier that permits the transport of certain species across it from one fluid to another. Membranes can be classified by the operating driving force for transport.
Hydrostatic Pressure
Microfiltration
The microfiltration membrane shown here is used in protein and nucleic acid concentration and purification.

General Information
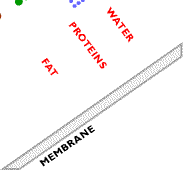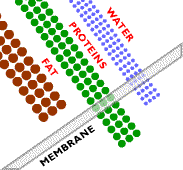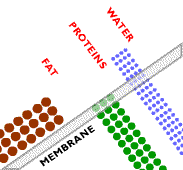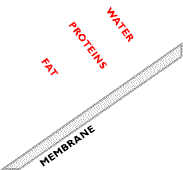
In microfiltration, a sieving effect separates particles based on their size. A mixture of components of different sizes is brought to the surface of a semipermeable membrane, meaning the membrane only allows certain species to permeate through. Under the driving force of a hydrostatic pressure gradient, some of the components permeate the membrane, whereas others do not, resulting in a separation.

Equipment Design
A symmetric microporous membrane (a membrane consisting of uniform pore diameters) is used to separate the components in microfiltration systems, such as the one shown here. Microporous membranes consist of a solid matrix with holes or pores. In microfiltration, these pores have a diameter between 0.1 and 10 micrometers.
(Copyright Copyright GEA Process Engineering, www.niroinc.com)
In separations involving straight- through filters, known as dead-end filtration, all the feed solution is forced through the membrane by an applied pressure. Essentially all the fluid permeates the membrane in one pass. For applications with high solid concentration, plugs may occur.
Microfiltration membranes can also be operated in a crossflow mode, where the feed solution is pumped across the membrane parallel to its surface. By maintaining a high velocity across the membrane, the retained solid is swept off the surface of the membrane, as shown below. This makes the crossflow mode ideal when a significant amount of material would be retained on the membrane. However, this mode of separation is not very efficient, and a recycling loop might be necessary.
Usage Examples
Microfiltration is used for process water purification and wastewater treatment. The microfiltration membrane system shown here is used for wastewater treatment.

Microfiltration membranes are also used in a wide range of medical applications, such as diagnostics, filtration, controlled release applications, and electromechanical cell components. Microfiltration systems can be also used in multiple industries such as the pharmaceutical, food, and dairy industries. The pilot plant microfiltration system shown below will be used for bacteria and fat removal from skim milk or whey. It will provide accurate results that can be replicated for full-scale production.

Advantages
- Effective in separating small particles of differing sizes or molecular mass.
Disadvantages
- Ineffective in separating particles of similar size or molecular mass.
- Salts and macromolecules are allowed to pass through the membrane.
- Dead-end filters may plug.
- Cross-flow filters are inefficient, requiring to recycle loops.
Ultrafiltration
Ultrafiltration systems, such as the one shown here, are different from microfiltration systems only in that they use membranes with smaller pores.

General Information
Ultrafiltration involves the use of a sieving mechanism to separate components. The difference between ultrafiltration and microfiltration is in the particle size of the components that can be filtered: ultrafiltration retains particles smaller than 0.1 micrometers in diameter. Below are examples of spiral wound ultrafiltration system membranes. On the left are polymeric membranes and on the right are examples of stainless steel and ceramic membranes.


(Copyright Copyright GEA Process Engineering, www.niroinc.com)
Equipment Design
The picture below shows an ultrafiltration membrane system. Ultrafiltration membranes are asymmetric microporous membranes, meaning the pore diameter increases from one side of the membrane to the other. These membranes are usually made from polymers. Most ultrafiltration processes operate in crossflow mode, as described in the Microfiltration section above. There are three main types of ultrafiltration membranes: spiral, tubular, and hollow fiber.
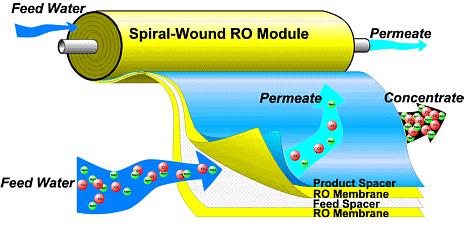
Spiral membranes are produced by winding the membrane around a perforated center tube, where permeate is collected. Feedwater is purified when it passes through one layer of the membrane and flows into the permeate tube. Spiral membranes offer the advantages of a wide range of membrane dimensions, lower energy costs due to reduced pumping requirements, and higher packing density. They can also be operated at elevated pressure and temperature.
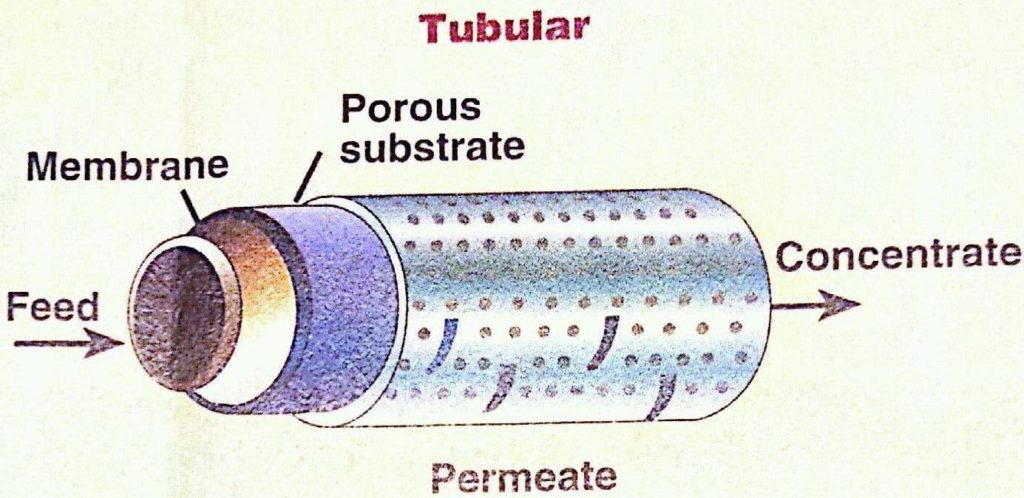
Tubular membranes consist of a support tube with a membrane cast on the inside. These tubes are often bundled into modules, such as those shown below. Inside diameters typically range from 1/4 inch up to 1 inch. The membrane is usually coated on the inside of the tube. The feed solution flows through the interior from one end to the other. The permeate passes through the membrane and is collected on the outside of the tube. These membranes can be made of several different materials including ceramic, carbon, stainless steel, and various thermoplastics. The tubular design is particularly useful for operations involving high solids concentrations, since plugging is kept to a minimum, and the product recovery is high. This design can be used in virtually every industrial application. Shown below are some examples of tubular membranes as well as a diagram of a tubular membrane.
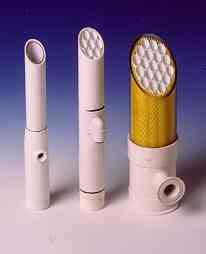
(Copyright GEA Process Engineering, www.niroinc.com)
Hollow fiber membranes, also called capillary membranes, allow a high membrane surface area to be contained in a compact module, providing high capacity. These membranes have an overall smaller inner tube diameter than tubular membranes and consist of unsupported membrane polymers. Such polymers require rigid support on each end of the tube. This support is provided by an epoxy potting of a bundle of the fibers inside. Each hollow fiber has a diameter of about 0.5 millimeters. The feed flow can go down the interior of the fibers, or around their outside. The diagram below shows the different sections of a hollow, or capillary, membrane and the picture below shows examples of different capillary membranes.
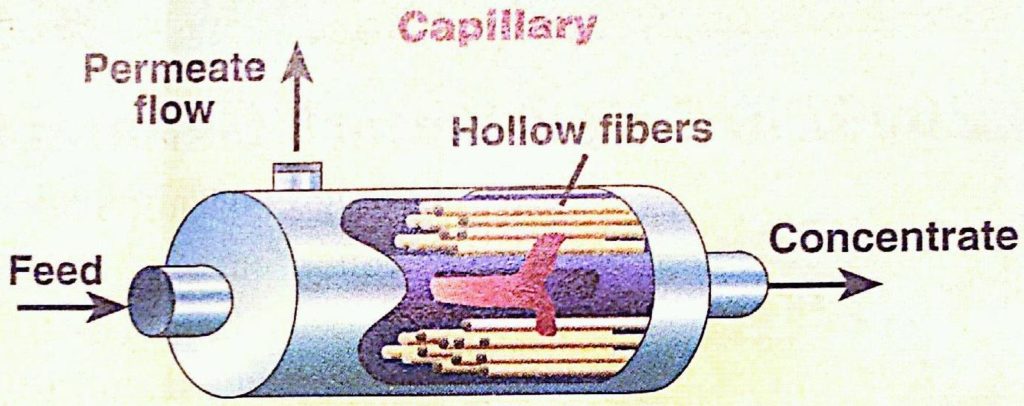

Usage Examples
Ultrafiltration is used in many applications, ranging from the treatment of industrial effluents and process streams to separation, concentration, and purification of pharmaceutical. The spiral wound ultrafiltration system shown here is used in the concentration of whey.

Advantages
- Effective in separating small particles of different sizes.
- Separates smaller-sized particles than microfiltration.
Disadvantages
- Ineffective in separating particles of similar size.
Reverse Osmosis
In reverse osmosis, pressure is applied to separate water from a salt solution.

General Information
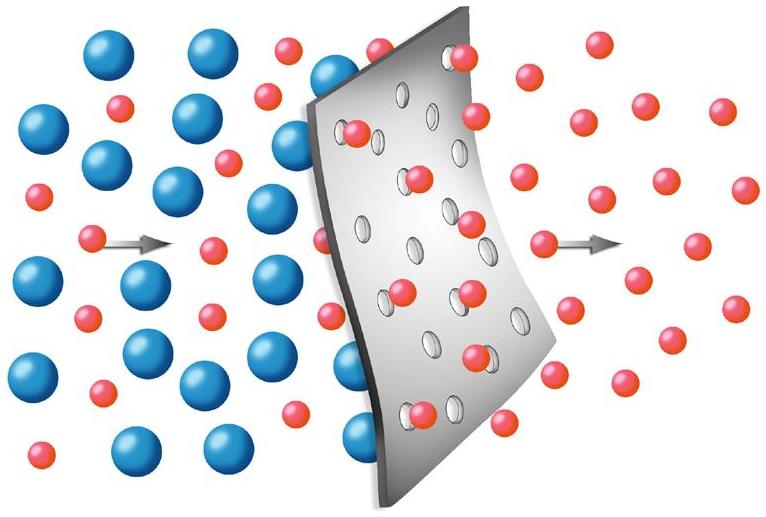
In traditional osmosis (Figure 2), water from the high water concentration side of the membrane will naturally diffuse through to the low water concentration side to tend toward equilibrium. The concentration difference generates an osmotic pressure that forces the water to flow from high to low concentration. In reverse osmosis (Figure 3), particles, macromolecules, and low molecular mass compounds are separated from a solvent, usually water. An overpressure creates pressure-driven flow through the membrane from low to high concentration, which allows for a high water purity to be achieved. This overpressure needs to be strong enough to overcome the osmotic pressure in the system.
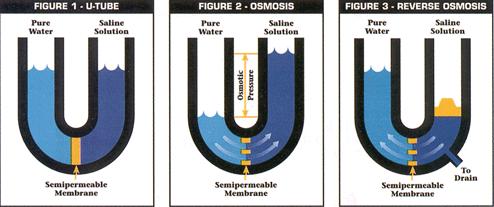
Reverse osmosis systems like the ones in the pictures below are primarily used to remove ionic salts, organic compounds, and other impurities from water.
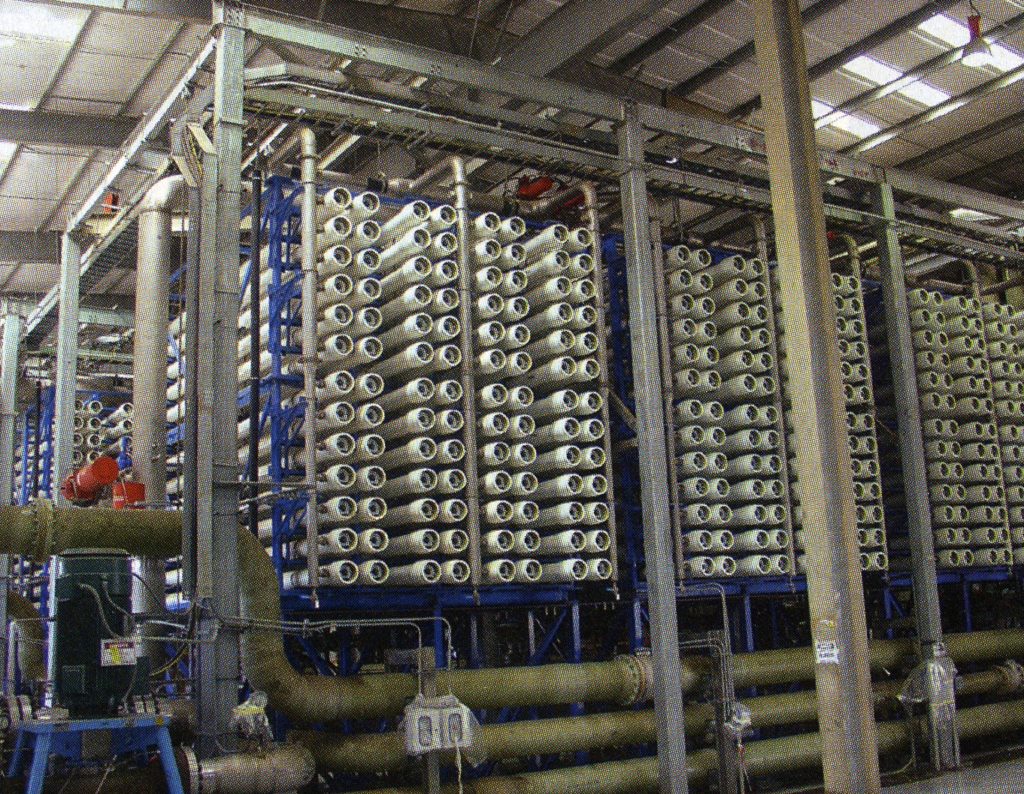

Equipment Design
Three major factors that affect reverse osmosis efficiency are membranes, pumps, and energy recovery devices. Membranes should have a high water permeability and a low salt permeability, a large surface area, a low-pressure drop across the membrane, and be very thin and defect-free. Thin-film composite (TFC) membranes are used in most cases since they possess many of these qualities. High-pressure pumps are often used to generate flow for feed solutions, and sometimes they are also used to introduce crossflow. Circulation pumps can be used to create the crossflow, which reduces the pressure drop needed at the high-pressure pump. Booster pumps can be added to later stages to counteract the increase in osmotic pressure through the system. Energy recovery devices are mainly found in seawater operations. They regain some of the hydraulic energy generated in the system so that it can be used to dispose of brine and collect more feedwater.
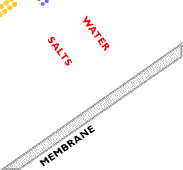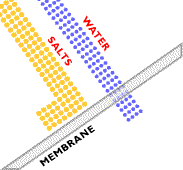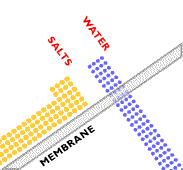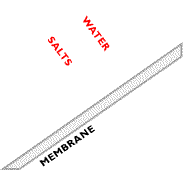
(Copyright GEA Process Engineering, www.niroinc.com)
Below is an animation of normal flow reverse osmosis, the stream to be purified enters through the top inlet and a piston forces the liquid through the membrane (blue). The particles are then discarded. Reverse osmosis can also be operated using crossflow, where the feed solution is pumped across the membrane parallel to its surface.
Usage Examples
Reverse osmosis is most widely used in water purification. Usually, this process involves removing salts from seawater. Other applications include the production of industrial process water, water for medical use, and the purification of water for use in the steam cycle of coal-fired power plants. The picture below to the left is an example of an under-counter reverse osmosis system used to provide clean tap water, like that shown to the right.


(Copyright Excel Water Technologies Inc., Ft. Lauderdale, FL)
Reverse osmosis is also used to produce ultrapure water for the fabrication of microelectronics such as semiconductors, flat panel displays, and disk drives. In addition, reverse osmosis can also be used in water softening systems, such as to enhance soap performance in a car wash. The reverse osmosis systems shown below can be used to purify water for entire households or for multiple commercial and industrial applications such as pharmaceuticals and food processing.


(Copyright Excel Water Technologies Inc., Ft. Lauderdale, FL)
Advantages
- Effective in the desalinization of water.
- Low replacement costs, long lifetime if operated properly.
- Reverse osmosis can be operated at high pH values to reduce fouling and provide higher recovery.
- Reliable method of water purification.
- Less expensive than more traditional water production methods.
Disadvantages
- There is often a high-pressure requirement to counter the osmotic pressure of the system.
- Costs involving brine disposal and energy consumption are high.
- Membrane fouling can occur.
- Pretreatment of the feedwater may be necessary to reduce the concentration of particles that cause fouling.
- Posttreatment is needed to dispose of undesired brine at the end of the process.
Gas Separation
In membrane-based gas separation, a membrane is used to separate a mixture of gases. The membrane shown below can separate carbon dioxide from flue gases for re-use.

General Information
In gas separation membrane systems, one or more species in a gas stream are isolated. A membrane acts as a molecular-scale filter, separating the mixture. There are four types of gas separation membranes: Knudsen membranes, ultramicroporous membranes, solution-diffusion membranes, and ion transport membranes. The picture below is an example of a laboratory-scale membrane used to separate hydrogen from hydrocarbon gas mixtures.
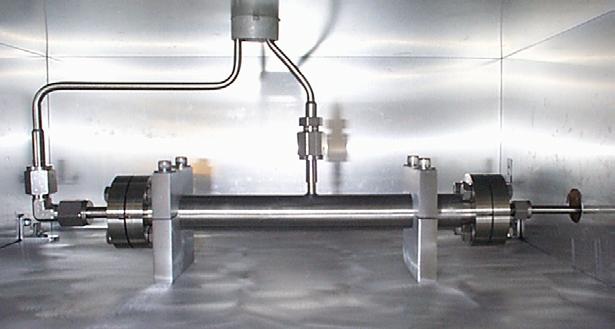
Equipment Design
The selectivity of a membrane quantifies its ability to remove the desired component from a mixture. Knudsen diffusion membranes have low selectivities, resulting in inefficient separation. Pores in the barrier layer of these membranes are smaller in diameter than the distance a molecule would travel between collisions. Smaller particles, such as hydrogen, bounce through the pore whereas most (but not all) of the larger molecules do not. This produces a low separation factor.
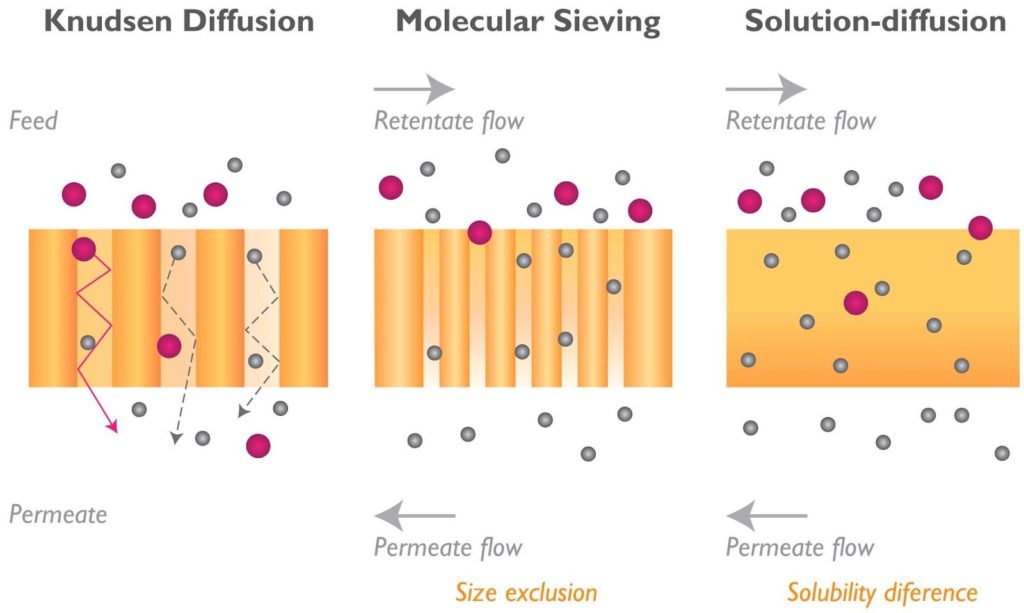
Molecular-sieve membranes, or ultramicroporous membranes, separate molecules based on size. They have a continuous network of passages connecting the upstream and downstream faces of the membrane. These pores must be smaller than a certain critical size to be effective and must remain open for continuous flow to the downstream side of the membrane.
In solution-diffusion membranes, gas dissolves in the membrane material and diffuses from high concentration to low concentration. Since there is a higher concentration on the feed side of the membrane, the gas diffuses to the permeate side. Certain gases permeate the membrane, whereas others do not, resulting in a separation.
Ion transport membranes are made of a ceramic material that ionizes under pressure and temperature. The oxygen molecules are separated from the gas when they form ionic bonds with the ionized membrane material. The ion transport membrane separates oxygen using less energy since it requires no external electrical source.
Usage Examples
Examples of isolating pure gases from a mixture include refinery hydrogen recovery, carbon dioxide recovery from well injections and hydrogen sulfide removal from sour gas. The picture shown below is a hydrogen-powered car. Certain microporous membranes can be used to separate hydrogen from mixed gas streams, allowing them to be used to power cars.

Advantages
- Effective in separating a gas mixture of unwanted components
Disadvantages
- Low selectivity
Vapor Pressure
Pervaporation
General Information/Equipment Design
In evaporation, a nonporous membrane is used to separate miscible liquid mixtures into two concentrated streams. A vacuum is applied to the permeate side of the membrane. This keeps the partial pressure of the permeate lower than the saturation pressure, producing the driving force for separation. The permeate is removed as vapor and then condensed.
Usage Examples
Pervaporation is used primarily in the dehydration of organic solvents, as well as in wastewater treatment.
Membrane Distillation
General Information/Equipment Design
Membrane distillation is very similar to pervaporation in that the permeate exits as a vapor, and a pressure differential supplies the driving force. A porous membrane is used to separate a liquid mixture by vaporization at the mouths of the pores, followed by vapor transport through the pores. The vapor permeate is then condensed by a cooled surface placed relatively close to the membrane. In the animation shown here, the feed solution is flowing down and the stream of cooling water is flowing upwards.
Usage Examples
Membrane distillation is used for water purification and demineralization of seawater, brackish water, somewhat salty water, and wastewater.
Electrical Potential
Electrodialysis
In electrodialysis, an electric charge is used to separate ionic species from a solution.

General Information
In electrodialysis, electrically charged membranes and an electrical potential difference are used to separate ionic components of an aqueous solution. The picture below shows the preparation of anion and cation membranes.

Equipment Design
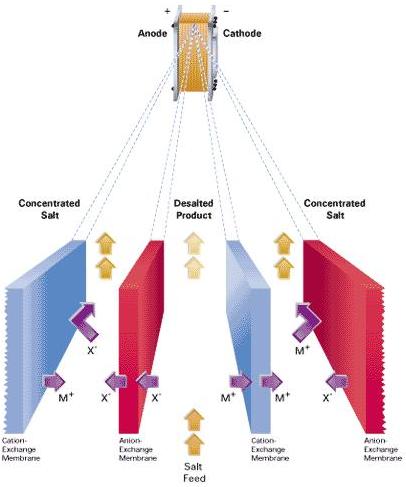
The electrodialysis stack consists of the alternating anion (red) and cation (blue) exchange membranes used to separate salt solutions. These membranes only allow ions with the corresponding charges to pass (e.g., anions pass through anion exchange membranes). The driving force is an electrically applied direct current flowing from an anode to a cathode.
A salt solution is fed into the electrodialysis stack. The current causes cations to migrate towards the cathode and anions towards the anode. Upon contacting an ion exchange membrane, the corresponding ions pass, whereas others are rejected. This yields a desalted product and concentrated salts.
Usage Examples
Electrodialysis is used in the desalting and purification of organic acids and solvents, desalting of foods, deacidification of foods, and wastewater minimization. A process very similar to electrodialysis, water splitting, is common in organic and amino acids production. In water-splitting an ion exchange membrane separates ionic species, while a bipolar membrane splits water into hydrogen and hydroxide ions. Below is a picture of bipolar membrane electrodialysis stacks used for the production of acetic acid.

Advantages
- High selectivity for charged components
- Lower energy and investment costs
- Continuous operation
- High product recovery rates
Disadvantages
- Only works with charged species
Concentration
Dialysis
General Information
Dialysis is a membrane process driven by a solute concentration difference between the feed side of the membrane and the permeate side, also known as the dialysate side. Components diffuse through the selectively permeable membrane. The membranes shown here are used in the concentration of dilute antibodies, enzymes, and viruses.
Usage Examples
Dialysis is used in the medical field in plasma purification and in artificial kidneys. Below is an illustration of the artificial kidney. Blood, shown in red, flows through a system of tubes composed of selectively permeable membranes. Dialysis fluid, with a composition similar to that of blood but with a low waste concentration, flows in the opposite direction, shown in blue. The waste diffuses from the blood to the dialysate fluid.
Advantages
- Simple process
- Avoids the cost associated with high pressure
Disadvantages
- Relatively slow process
- Limited degree of selectivity
Facilitated Transport
General Information/Equipment Design
Facilitated transport involves chemical reactions in the separation of a mixture. The membrane contains selective carriers that react reversibly with one of the components, but not the other. The reacting component will be absorbed in the membrane, and transported from the higher concentration side to the lower concentration one. The reverse reaction then takes place, releasing the separated component from the membrane on the permeate side.
Acknowledgements
- Cooperative Research Centre for Greenhouse Gas Technologies (CO2CRC), Australia
- Eurodia Industrie SA, Rungis Cedex, France
- Excel Water Technologies Inc., Ft. Lauderdale, FL
- GEA Process Engineering
- Millipore Corporation, Billerica, MA
- Oak Ridge National Laboratory
- Sterlitech Corporation, Kent, WA
References
- Baker, R.W., E.L. Cussler. W. Eykamp, W.J. Koros, R.L. Riley, and H. Strathmann. Membrane Separation Systems. New Jersey: Noyes Data Corporation, 1991. Print.
- LePree, Joy. “Membranes For Gas Separation” Chemical Engineering. February 2012: 17-20. Print.
- Mcllvaine, Robert. “Reverse Osmosis” Chemical Engineering. August 2008: 20-24. Print.
- Perry, Robert H. and Don W. Green. Perry’s Chemical Engineers Handbook. 7th ed. New York: Mcgraw-Hill, 1997: 22-42 – 22-69. Print.
- Schweitzer, Philip A. Handbook of Separation Techniques for Chemical Engineers. 3rd ed. New York: McGraw-Hill: 1997. Print.
- Scott, K., and R. Hughes. Industrial Membrane Separation Technology. New York: Chapman and Hall, 1996. Print.
- Seeley, Rod R., Trent D. Stephens, and Philip Tate. Anatomy & Physiology, 2nd ed. St. Louis, MO: Mosby-Year Book, 1992. Print.
- Stover, Richard. “A Primer on Reverse Osmosis Technology” Chemical Engineering. July 2014: 38-44. Print.
- Torzewski, Kate. “Facts at your Fingerprints: Membrane Configurations” Chemical Engineering March 2009: 27. Print.
- Turner, M.K. Effective Industrial Membrane Processes: Benefits and Opportunities. New York: Elsevier Applied Science, 1991. Print.
- Winston Ho, W.S. and Kamalesh K. Sirkar. Membrane Handbook. New York: Van Nostrand Reinhold, 1992. Print.
Developers
- Jeff Scramlin
- Brad Lintner
- Alex Wozniac
- Joseph Palazzolo
- Abigail Nalbandian
- Kelsey Kaplan
- Keith Minbiole
- Eric Giuffrida
- Thomas Plegue
- Michael Andrews